If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.

Properties of matter (Essentials) - Class 11th
Course: properties of matter (essentials) - class 11th > unit 1, properties of matter (course intro).
Want to join the conversation?
Video transcript.
- STEM Ambassadors
- School trusts
- ITE and governors
- Invest in schools
- STEM careers inspiration
- Benefits and impact
- Our supporters
- Become a STEM Ambassador
- Request a STEM Ambassador
- Employer information
- Training and support
- STEM Ambassadors Partners
- Working with community groups
- Search icon
- Join the STEM Community
Mechanical properties of matter
- stress, strain, Young modulus
- force-extension graphs, energy stored
For many students, this topic will be the first time in physics they have been asked to explicitly link microscale structure (molecular bonds) with observed behaviour (stiffness and other characteristics). Their familiarity with the language used will vary depending on previous study, which will include design and technology, engineering and science. It's often worth spending a little time making sure that everyone is happy using the scientific terms precisely, as many of them have everyday uses which are not quite correct. You may like to remind them that they already know the terms mass, weight and gravity are used in a very particular way by physicists; the terms stiffness, stress and strength are the same. A possible structure might include:
- to recap Hooke's Law and other previous work done describing the behaviour of materials that stretch
- having students calculate stiffness of lab springs/materials using obtained data and apply experimental language to their work
- being precise when introducing and defining the terms stress and strain . You can show how they are related by the Young modulus for a given material, and how this is independent of the shape of the sample
- checking understanding of mathematical relationships, units and symbols is a good opportunity to discuss techniques for revision, as well as having a clear physics benefit
- students taking practical measurements to calculate Young modulus and compare this to accepted values
- discussing the relevance of material characteristics to their uses, being sure to include a range of applications from prosthetics, sports equipment design and consumer products as well as civil engineering
- students are often keen to use simulations and computer models in design and analysis; discussions of the benefits and limitations of this can be illuminating
- students being confident with the derivation and calculation of energy stored by a spring and knowing they must be able to find an approximate answer by using the area under a force-extension graph
Whilst this list provides a source of information and ideas for experimental work, it is important to note that recommendations can date very quickly. Do NOT follow suggestions which conflict with current advice from CLEAPSS or recent safety guides. eLibrary users are responsible for ensuring that any activity, including practical work, which they carry out is consistent with current regulations related to Health and Safety and that they carry an appropriate risk assessment. Further information is provided in our Health and Safety guidance.
Episode 227: Hooke’s Law
Quality Assured Category: Science Publisher: Institute of Physics
Most students will have encountered Hooke's law before, as it is covered in most KS3 courses. Some will have revisited it during GCSE when it will have been linked to the idea of calculating elastic potential energy. The practical guidance here from the IoP focuses on reminding students of the measurements to be made and the calculations that can then be performed.
Some of the practical work described may be familiar to students as stretching carrier bags has featured in GCSE assessed practical work several times. The terms are carefully distinguished and the idea of springs in series and parallel is introduced as - excuse the pun - extension.

Mechanics of Elastic Solids
An informative website with many material and mechanical science explanations.
Stretching copper wire
In this classic experiment students learn how to measure a small change in length accurately which gives rise to interesting discussions about the challenges faced by experimental physicists.
Episode 228: The Young Modulus
Another IoP resource (the complete mechanics set is on the eLibrary ) this moves on from Hooke's law to the relationship between stress and strain. Different practical approaches are compared and student materials include guidance on creating and interpreting stress/strain graphs. Sample data is supplied for various materials.
Of particular interest to most colleagues will be the suggestions for different ways to measure extension during the practicals. This would be an excellent stimulus for discussion about experimental methods in general and the application of scientific language to a particular practical.
Modulus of Elasticity
This page is one from the School Physics website created by an experienced teacher and author, Keith Gibbs. It is intended for students to access independently and is a useful explanation of the three main ways in which 'modulus of elasticity' may be used in textbooks. In most cases the Young modulus is intended but for those with an interest in engineering the understanding of other approaches will be useful. Be clear with students about what concepts they will need for their exam.
Masses and Springs
This simulation of a spring being stretched by adding masses allows students to change the stiffness of the spring as well as the strength of gravity. Results can be collected, plotted and compared to those from 'hands-on' practicals. In itself, this may prompt a useful discussion of the value of scientific modelling. You may choose to set tasks using this as preparation for the practical lesson, or as a follow-up to consolidate their knowledge. The reference to damping will be useful when you cover oscillations and the discussion of energy will help link the topics together.
Elastic Energy
Hyper Physics explains key concepts in physics, this webpage covers elastic potential energy.
The Study of Matter
A collection of short discussion pieces about the role of materials science in teaching, it may provide useful contexts and anecdotes for what we teach our students. It is interesting to see how quickly some parts have become almost standard, for example the discussion on page 38 about 'virtual' materials testing.
- TPC and eLearning
- What's NEW at TPC?
- Read Watch Interact
- Practice Review Test
- Teacher-Tools
- Subscription Selection
- Seat Calculator
- Ad Free Account
- Edit Profile Settings
- Classes (Version 2)
- Student Progress Edit
- Task Properties
- Export Student Progress
- Task, Activities, and Scores
- Metric Conversions Questions
- Metric System Questions
- Metric Estimation Questions
- Significant Digits Questions
- Proportional Reasoning
- Acceleration
- Distance-Displacement
- Dots and Graphs
- Graph That Motion
- Match That Graph
- Name That Motion
- Motion Diagrams
- Pos'n Time Graphs Numerical
- Pos'n Time Graphs Conceptual
- Up And Down - Questions
- Balanced vs. Unbalanced Forces
- Change of State
- Force and Motion
- Mass and Weight
- Match That Free-Body Diagram
- Net Force (and Acceleration) Ranking Tasks
- Newton's Second Law
- Normal Force Card Sort
- Recognizing Forces
- Air Resistance and Skydiving
- Solve It! with Newton's Second Law
- Which One Doesn't Belong?
- Component Addition Questions
- Head-to-Tail Vector Addition
- Projectile Mathematics
- Trajectory - Angle Launched Projectiles
- Trajectory - Horizontally Launched Projectiles
- Vector Addition
- Vector Direction
- Which One Doesn't Belong? Projectile Motion
- Forces in 2-Dimensions
- Being Impulsive About Momentum
- Explosions - Law Breakers
- Hit and Stick Collisions - Law Breakers
- Case Studies: Impulse and Force
- Impulse-Momentum Change Table
- Keeping Track of Momentum - Hit and Stick
- Keeping Track of Momentum - Hit and Bounce
- What's Up (and Down) with KE and PE?
- Energy Conservation Questions
- Energy Dissipation Questions
- Energy Ranking Tasks
- LOL Charts (a.k.a., Energy Bar Charts)
- Match That Bar Chart
- Words and Charts Questions
- Name That Energy
- Stepping Up with PE and KE Questions
- Case Studies - Circular Motion
- Circular Logic
- Forces and Free-Body Diagrams in Circular Motion
- Gravitational Field Strength
- Universal Gravitation
- Angular Position and Displacement
- Linear and Angular Velocity
- Angular Acceleration
- Rotational Inertia
- Balanced vs. Unbalanced Torques
- Getting a Handle on Torque
- Torque-ing About Rotation
- Properties of Matter
- Fluid Pressure
- Buoyant Force
- Sinking, Floating, and Hanging
- Pascal's Principle
- Flow Velocity
- Bernoulli's Principle
- Balloon Interactions
- Charge and Charging
- Charge Interactions
- Charging by Induction
- Conductors and Insulators
- Coulombs Law
- Electric Field
- Electric Field Intensity
- Polarization
- Case Studies: Electric Power
- Know Your Potential
- Light Bulb Anatomy
- I = ∆V/R Equations as a Guide to Thinking
- Parallel Circuits - ∆V = I•R Calculations
- Resistance Ranking Tasks
- Series Circuits - ∆V = I•R Calculations
- Series vs. Parallel Circuits
- Equivalent Resistance
- Period and Frequency of a Pendulum
- Pendulum Motion: Velocity and Force
- Energy of a Pendulum
- Period and Frequency of a Mass on a Spring
- Horizontal Springs: Velocity and Force
- Vertical Springs: Velocity and Force
- Energy of a Mass on a Spring
- Decibel Scale
- Frequency and Period
- Closed-End Air Columns
- Name That Harmonic: Strings
- Rocking the Boat
- Wave Basics
- Matching Pairs: Wave Characteristics
- Wave Interference
- Waves - Case Studies
- Color Addition and Subtraction
- Color Filters
- If This, Then That: Color Subtraction
- Light Intensity
- Color Pigments
- Converging Lenses
- Curved Mirror Images
- Law of Reflection
- Refraction and Lenses
- Total Internal Reflection
- Who Can See Who?
- Formulas and Atom Counting
- Atomic Models
- Bond Polarity
- Entropy Questions
- Cell Voltage Questions
- Heat of Formation Questions
- Reduction Potential Questions
- Oxidation States Questions
- Measuring the Quantity of Heat
- Hess's Law
- Oxidation-Reduction Questions
- Galvanic Cells Questions
- Thermal Stoichiometry
- Molecular Polarity
- Quantum Mechanics
- Balancing Chemical Equations
- Bronsted-Lowry Model of Acids and Bases
- Classification of Matter
- Collision Model of Reaction Rates
- Density Ranking Tasks
- Dissociation Reactions
- Complete Electron Configurations
- Elemental Measures
- Enthalpy Change Questions
- Equilibrium Concept
- Equilibrium Constant Expression
- Equilibrium Calculations - Questions
- Equilibrium ICE Table
- Intermolecular Forces Questions
- Ionic Bonding
- Lewis Electron Dot Structures
- Limiting Reactants
- Line Spectra Questions
- Mass Stoichiometry
- Measurement and Numbers
- Metals, Nonmetals, and Metalloids
- Metric Estimations
- Metric System
- Molarity Ranking Tasks
- Mole Conversions
- Name That Element
- Names to Formulas
- Names to Formulas 2
- Nuclear Decay
- Particles, Words, and Formulas
- Periodic Trends
- Precipitation Reactions and Net Ionic Equations
- Pressure Concepts
- Pressure-Temperature Gas Law
- Pressure-Volume Gas Law
- Chemical Reaction Types
- Significant Digits and Measurement
- States Of Matter Exercise
- Stoichiometry Law Breakers
- Stoichiometry - Math Relationships
- Subatomic Particles
- Spontaneity and Driving Forces
- Gibbs Free Energy
- Volume-Temperature Gas Law
- Acid-Base Properties
- Energy and Chemical Reactions
- Chemical and Physical Properties
- Valence Shell Electron Pair Repulsion Theory
- Writing Balanced Chemical Equations
- Mission CG1
- Mission CG10
- Mission CG2
- Mission CG3
- Mission CG4
- Mission CG5
- Mission CG6
- Mission CG7
- Mission CG8
- Mission CG9
- Mission EC1
- Mission EC10
- Mission EC11
- Mission EC12
- Mission EC2
- Mission EC3
- Mission EC4
- Mission EC5
- Mission EC6
- Mission EC7
- Mission EC8
- Mission EC9
- Mission RL1
- Mission RL2
- Mission RL3
- Mission RL4
- Mission RL5
- Mission RL6
- Mission KG7
- Mission RL8
- Mission KG9
- Mission RL10
- Mission RL11
- Mission RM1
- Mission RM2
- Mission RM3
- Mission RM4
- Mission RM5
- Mission RM6
- Mission RM8
- Mission RM10
- Mission LC1
- Mission RM11
- Mission LC2
- Mission LC3
- Mission LC4
- Mission LC5
- Mission LC6
- Mission LC8
- Mission SM1
- Mission SM2
- Mission SM3
- Mission SM4
- Mission SM5
- Mission SM6
- Mission SM8
- Mission SM10
- Mission KG10
- Mission SM11
- Mission KG2
- Mission KG3
- Mission KG4
- Mission KG5
- Mission KG6
- Mission KG8
- Mission KG11
- Mission F2D1
- Mission F2D2
- Mission F2D3
- Mission F2D4
- Mission F2D5
- Mission F2D6
- Mission KC1
- Mission KC2
- Mission KC3
- Mission KC4
- Mission KC5
- Mission KC6
- Mission KC7
- Mission KC8
- Mission AAA
- Mission SM9
- Mission LC7
- Mission LC9
- Mission NL1
- Mission NL2
- Mission NL3
- Mission NL4
- Mission NL5
- Mission NL6
- Mission NL7
- Mission NL8
- Mission NL9
- Mission NL10
- Mission NL11
- Mission NL12
- Mission MC1
- Mission MC10
- Mission MC2
- Mission MC3
- Mission MC4
- Mission MC5
- Mission MC6
- Mission MC7
- Mission MC8
- Mission MC9
- Mission RM7
- Mission RM9
- Mission RL7
- Mission RL9
- Mission SM7
- Mission SE1
- Mission SE10
- Mission SE11
- Mission SE12
- Mission SE2
- Mission SE3
- Mission SE4
- Mission SE5
- Mission SE6
- Mission SE7
- Mission SE8
- Mission SE9
- Mission VP1
- Mission VP10
- Mission VP2
- Mission VP3
- Mission VP4
- Mission VP5
- Mission VP6
- Mission VP7
- Mission VP8
- Mission VP9
- Mission WM1
- Mission WM2
- Mission WM3
- Mission WM4
- Mission WM5
- Mission WM6
- Mission WM7
- Mission WM8
- Mission WE1
- Mission WE10
- Mission WE2
- Mission WE3
- Mission WE4
- Mission WE5
- Mission WE6
- Mission WE7
- Mission WE8
- Mission WE9
- Vector Walk Interactive
- Name That Motion Interactive
- Kinematic Graphing 1 Concept Checker
- Kinematic Graphing 2 Concept Checker
- Graph That Motion Interactive
- Two Stage Rocket Interactive
- Rocket Sled Concept Checker
- Force Concept Checker
- Free-Body Diagrams Concept Checker
- Free-Body Diagrams The Sequel Concept Checker
- Skydiving Concept Checker
- Elevator Ride Concept Checker
- Vector Addition Concept Checker
- Vector Walk in Two Dimensions Interactive
- Name That Vector Interactive
- River Boat Simulator Concept Checker
- Projectile Simulator 2 Concept Checker
- Projectile Simulator 3 Concept Checker
- Hit the Target Interactive
- Turd the Target 1 Interactive
- Turd the Target 2 Interactive
- Balance It Interactive
- Go For The Gold Interactive
- Egg Drop Concept Checker
- Fish Catch Concept Checker
- Exploding Carts Concept Checker
- Collision Carts - Inelastic Collisions Concept Checker
- Its All Uphill Concept Checker
- Stopping Distance Concept Checker
- Chart That Motion Interactive
- Roller Coaster Model Concept Checker
- Uniform Circular Motion Concept Checker
- Horizontal Circle Simulation Concept Checker
- Vertical Circle Simulation Concept Checker
- Race Track Concept Checker
- Gravitational Fields Concept Checker
- Orbital Motion Concept Checker
- Angular Acceleration Concept Checker
- Balance Beam Concept Checker
- Torque Balancer Concept Checker
- Aluminum Can Polarization Concept Checker
- Charging Concept Checker
- Name That Charge Simulation
- Coulomb's Law Concept Checker
- Electric Field Lines Concept Checker
- Put the Charge in the Goal Concept Checker
- Circuit Builder Concept Checker (Series Circuits)
- Circuit Builder Concept Checker (Parallel Circuits)
- Circuit Builder Concept Checker (∆V-I-R)
- Circuit Builder Concept Checker (Voltage Drop)
- Equivalent Resistance Interactive
- Pendulum Motion Simulation Concept Checker
- Mass on a Spring Simulation Concept Checker
- Particle Wave Simulation Concept Checker
- Boundary Behavior Simulation Concept Checker
- Slinky Wave Simulator Concept Checker
- Simple Wave Simulator Concept Checker
- Wave Addition Simulation Concept Checker
- Standing Wave Maker Simulation Concept Checker
- Color Addition Concept Checker
- Painting With CMY Concept Checker
- Stage Lighting Concept Checker
- Filtering Away Concept Checker
- InterferencePatterns Concept Checker
- Young's Experiment Interactive
- Plane Mirror Images Interactive
- Who Can See Who Concept Checker
- Optics Bench (Mirrors) Concept Checker
- Name That Image (Mirrors) Interactive
- Refraction Concept Checker
- Total Internal Reflection Concept Checker
- Optics Bench (Lenses) Concept Checker
- Kinematics Preview
- Velocity Time Graphs Preview
- Moving Cart on an Inclined Plane Preview
- Stopping Distance Preview
- Cart, Bricks, and Bands Preview
- Fan Cart Study Preview
- Friction Preview
- Coffee Filter Lab Preview
- Friction, Speed, and Stopping Distance Preview
- Up and Down Preview
- Projectile Range Preview
- Ballistics Preview
- Juggling Preview
- Marshmallow Launcher Preview
- Air Bag Safety Preview
- Colliding Carts Preview
- Collisions Preview
- Engineering Safer Helmets Preview
- Push the Plow Preview
- Its All Uphill Preview
- Energy on an Incline Preview
- Modeling Roller Coasters Preview
- Hot Wheels Stopping Distance Preview
- Ball Bat Collision Preview
- Energy in Fields Preview
- Weightlessness Training Preview
- Roller Coaster Loops Preview
- Universal Gravitation Preview
- Keplers Laws Preview
- Kepler's Third Law Preview
- Charge Interactions Preview
- Sticky Tape Experiments Preview
- Wire Gauge Preview
- Voltage, Current, and Resistance Preview
- Light Bulb Resistance Preview
- Series and Parallel Circuits Preview
- Thermal Equilibrium Preview
- Linear Expansion Preview
- Heating Curves Preview
- Electricity and Magnetism - Part 1 Preview
- Electricity and Magnetism - Part 2 Preview
- Vibrating Mass on a Spring Preview
- Period of a Pendulum Preview
- Wave Speed Preview
- Slinky-Experiments Preview
- Standing Waves in a Rope Preview
- Sound as a Pressure Wave Preview
- DeciBel Scale Preview
- DeciBels, Phons, and Sones Preview
- Sound of Music Preview
- Shedding Light on Light Bulbs Preview
- Models of Light Preview
- Electromagnetic Radiation Preview
- Electromagnetic Spectrum Preview
- EM Wave Communication Preview
- Digitized Data Preview
- Light Intensity Preview
- Concave Mirrors Preview
- Object Image Relations Preview
- Snells Law Preview
- Reflection vs. Transmission Preview
- Magnification Lab Preview
- Reactivity Preview
- Ions and the Periodic Table Preview
- Periodic Trends Preview
- Intermolecular Forces Preview
- Melting Points and Boiling Points Preview
- Bond Energy and Reactions Preview
- Reaction Rates Preview
- Ammonia Factory Preview
- Stoichiometry Preview
- Nuclear Chemistry Preview
- Gaining Teacher Access
- Tasks and Classes
- Tasks - Classic
- Subscription
- Subscription Locator
- 1-D Kinematics
- Newton's Laws
- Vectors - Motion and Forces in Two Dimensions
- Momentum and Its Conservation
- Work and Energy
- Circular Motion and Satellite Motion
- Thermal Physics
- Static Electricity
- Electric Circuits
- Vibrations and Waves
- Sound Waves and Music
- Light and Color
- Reflection and Mirrors
- About the Physics Interactives
- Task Tracker
- Usage Policy
- Newtons Laws
- Vectors and Projectiles
- Forces in 2D
- Momentum and Collisions
- Circular and Satellite Motion
- Balance and Rotation
- Electromagnetism
- Waves and Sound
- Atomic Physics
- Forces in Two Dimensions
- Work, Energy, and Power
- Circular Motion and Gravitation
- Sound Waves
- 1-Dimensional Kinematics
- Circular, Satellite, and Rotational Motion
- Einstein's Theory of Special Relativity
- Waves, Sound and Light
- QuickTime Movies
- About the Concept Builders
- Pricing For Schools
- Directions for Version 2
- Measurement and Units
- Relationships and Graphs
- Rotation and Balance
- Vibrational Motion
- Reflection and Refraction
- Teacher Accounts
- Task Tracker Directions
- Kinematic Concepts
- Kinematic Graphing
- Wave Motion
- Sound and Music
- About CalcPad
- 1D Kinematics
- Vectors and Forces in 2D
- Simple Harmonic Motion
- Rotational Kinematics
- Rotation and Torque
- Rotational Dynamics
- Electric Fields, Potential, and Capacitance
- Transient RC Circuits
- Light Waves
- Units and Measurement
- Stoichiometry
- Molarity and Solutions
- Thermal Chemistry
- Acids and Bases
- Kinetics and Equilibrium
- Solution Equilibria
- Oxidation-Reduction
- Nuclear Chemistry
- Newton's Laws of Motion
- Work and Energy Packet
- Static Electricity Review
- NGSS Alignments
- 1D-Kinematics
- Projectiles
- Circular Motion
- Magnetism and Electromagnetism
- Graphing Practice
- About the ACT
- ACT Preparation
- For Teachers
- Other Resources
- Solutions Guide
- Solutions Guide Digital Download
- Motion in One Dimension
- Work, Energy and Power
- Algebra Based Physics
- Other Tools
- Frequently Asked Questions
- Purchasing the Download
- Purchasing the CD
- Purchasing the Digital Download
- About the NGSS Corner
- NGSS Search
- Force and Motion DCIs - High School
- Energy DCIs - High School
- Wave Applications DCIs - High School
- Force and Motion PEs - High School
- Energy PEs - High School
- Wave Applications PEs - High School
- Crosscutting Concepts
- The Practices
- Physics Topics
- NGSS Corner: Activity List
- NGSS Corner: Infographics
- About the Toolkits
- Position-Velocity-Acceleration
- Position-Time Graphs
- Velocity-Time Graphs
- Newton's First Law
- Newton's Second Law
- Newton's Third Law
- Terminal Velocity
- Projectile Motion
- Forces in 2 Dimensions
- Impulse and Momentum Change
- Momentum Conservation
- Work-Energy Fundamentals
- Work-Energy Relationship
- Roller Coaster Physics
- Satellite Motion
- Electric Fields
- Circuit Concepts
- Series Circuits
- Parallel Circuits
- Describing-Waves
- Wave Behavior Toolkit
- Standing Wave Patterns
- Resonating Air Columns
- Wave Model of Light
- Plane Mirrors
- Curved Mirrors
- Teacher Guide
- Using Lab Notebooks
- Current Electricity
- Light Waves and Color
- Reflection and Ray Model of Light
- Refraction and Ray Model of Light
- Classes (Legacy Version)
- Teacher Resources
- Subscriptions

- Newton's Laws
- Einstein's Theory of Special Relativity
- About Concept Checkers
- School Pricing
- Newton's Laws of Motion
- Newton's First Law
- Newton's Third Law
- The Structure of Matter
- Neutral vs. Charged Objects
Not only do electrostatic occurrences permeate the events of everyday life, without the forces associated with static electricity, life as we know it would be impossible. Electrostatic forces - both attractive and repulsive in nature - hold the world of atoms and molecules together in perfect balance. Without this electric force, material things would not exist. Atoms as the building blocks of matter depend upon these forces. And material objects, including us Earthlings, are made of atoms and the acts of standing and walking, touching and feeling, smelling and tasting, and even thinking is the result of electrical phenomenon. Electrostatic forces are foundational to our existence.
One of the primary questions to be asked in this unit of The Physics Classroom is: How can an object be charged and what affect does that charge have upon other objects in its vicinity? The answer to this question begins with an understanding of the structure of matter. Understanding charge as a fundamental quantity demands that we have an understanding of the structure of an atom. So we begin this unit with what might seem to many students to be a short review of a unit from a Chemistry course.
History of Atomic Structure
The early Greeks were simply philosophers. They did not perform experiments to test their theories. In fact, science as an experimental discipline did not emerge as a credible and popular practice until sometime during the 1600s. So the search for the atom remained a philosophical inquiry for a couple of millennia. From the 1600s to the present century, the search for the atom became an experimental pursuit. Several scientists are notable; among them are Robert Boyle, John Dalton, J.J. Thomson, Ernest Rutherford, and Neils Bohr.
Boyle's studies (middle to late 1600s) of gaseous substances promoted the idea that there were different types of atoms known as elements. Dalton (early 1800s) conducted a variety of experiments to show that different elements can combine in fixed ratios of masses to form compounds. Dalton subsequently proposed one of the first theories of atomic behavior that was supported by actual experimental evidence.
English scientist J.J. Thomson's cathode ray experiments (end of the 19th century) led to the discovery of the negatively charged electron and the first ideas of the structure of these indivisible atoms. Thomson proposed the Plum Pudding Model , suggesting that an atom's structure resembles the favorite English dessert - plum pudding. The raisins dispersed amidst the plum pudding are analogous to negatively charged electrons immersed in a sea of positive charge.
Nearly a decade after Thomson, Ernest Rutherford's famous gold foil experiments led to the nuclear model of atomic structure. Rutherford's model suggested that the atom consisted of a densely packed core of positive charge known as the nucleus surrounded by negatively charged electrons. While the nucleus was unique to the Rutherford atom, even more surprising was the proposal that an atom consisted mostly of empty space. Most the mass was packed into the nucleus that was abnormally small compared to the actual size of the atom.
Neils Bohr improved upon Rutherford's nuclear model (1913) by explaining that the electrons were present in orbits outside the nucleus. The electrons were confined to specific orbits of fixed radius, each characterized by their own discrete levels of energy. While electrons could be forced from one orbit to another orbit, it could never occupy the space between orbits.
Bohr's view of quantized energy levels was the precursor to modern quantum mechanical views of the atoms. The mathematical nature of quantum mechanics prohibits a discussion of its details and restricts us to a brief conceptual description of its features. Quantum mechanics suggests that an atom is composed of a variety of subatomic particles. The three main subatomic particles are the proton, electron and neutron. The proton and neutron are the most massive of the three subatomic particles; they are located in the nucleus of the atom, forming the dense core of the atom. The proton is charged positively. The neutron does not possess a charge and is said to be neutral. The protons and neutrons are bound tightly together within the nucleus of the atom. Outside the nucleus are concentric spherical regions of space known as electron shells . The shells are the home of the negatively charged electrons. Each shell is characterized by a distinct energy level. Outer shells have higher energy levels and are characterized as being lower in stability. Electrons in higher energy shells can move down to lower energy shells; this movement is accompanied by the release of energy. Similarly, electrons in lower energy shells can be induced to move to the higher energy outer shells by the addition of energy to the atom. If provided sufficient energy, an electron can be removed from an atom and be freed from its attraction to the nucleus.
Application of Atomic Structure to Static Electricity
This brief excursion into the history of atomic theory leads to some important conclusions about the structure of matter that will be of utmost importance to our study of static electricity. Those conclusions are summarized here:
- All material objects are composed of atoms. There are different kinds of atoms known as elements; these elements can combine to form compounds. Different compounds have distinctly different properties. Material objects are composed of atoms and molecules of these elements and compounds, thus providing different materials with different electrical properties.
- An atom consists of a nucleus and a vast region of space outside the nucleus. Electrons are present in the region of space outside the nucleus. They are negatively charged and weakly bound to the atom. Electrons are often removed from and added to an atom by normal everyday occurrences. These occurrences are the focus of this Static Electricity unit of The Physics Classroom.
- The nucleus of the atom contains positively charged protons and neutral neutrons. These protons and neutrons are not removable or perturbable by usual everyday methods. It would require some form of high-energy nuclear occurrence to disturb the nucleus and subsequently dislodge its positively charged protons. These high-energy occurrences are fortunately not an everyday event and they are certainly not the subject of this unit of The Physics Classroom. One sure truth of this unit is that the protons and neutrons will remain within the nucleus of the atom. Electrostatic phenomenon can never be explained by the movement of protons.
A variety of phenomena will be pondered, investigated and explained through the course of this Static Electricity unit. Each phenomenon will be explained using a model of matter described by the above three statements. The phenomena will range from a rubber balloon sticking to a wooden door to the clinging together of clothes that have tumbled in the dryer to the bolt of lightning seen in the evening sky. Each of these phenomena will be explained in terms of electron movement - both within the atoms and molecules of a material and from the atoms and molecules of one material to those of another. In the next section of Lesson 1 we will explore how electron movement can be used to explain how and why objects acquire an electrostatic charge.
Check Your Understanding
Use your understanding of charge to answer the following questions. When finished, click the button to view the answers.
1. ____ are the charged parts of an atom.
a. Only electrons b. Only protons c. Neutrons only d. Electrons and neutrons e. Electrons and protons f. Protons and neutrons
Electrons are negatively charged and protons are positively charged. The neutrons do not have a charge.
- Triboelectric Charging
We store cookies data for a seamless user experience. To know more check the Privacy Policy
- computer-science
- engineering
- science-math
- Online Tutoring
- AI Essay Writer

- Management - Others
- FORMAL HOMEWORK EXERCISE Mechanics & Properties of...
FORMAL HOMEWORK EXERCISE Mechanics & Properties of Matter Attempt all 7 questions Homework is... 1 answer below »

11+ Users Viewed

2+ Downloaded Solutions

Florida, US Mostly Asked From
FORMAL HOMEWORK EXERCISE Mechanics & Properties of Matter Attempt all 7 questions Homework is due by Monday 15 December 1. A car has its tyres inflated to a pressure of 240 kPa on a day when the temperature is 5 °C. The car is then driven for several hours, and the temperature of the tyres is found to have risen to 35 °C. (a) Assuming the mass and volume of the air in the tyres has remained constant, calculate the new pressure in the tyres. (b) Explain why this happens to the pressure as the temperature rises, making reference to the kinetic theory of gases. 3 2. In preparation for a party, a balloon is inflated to a volume of 0.5 m in a cold room (0 °C). During the party, the room temperature rises to 22 °C. Calculate the new volume of the balloon, assuming the pressure inside it remains constant. 3. An astronaut on a spacewalk has an oxygen tank strapped to his suit. The oxygen in it is pressurised to 5 5 atmospheres (5.0 x 10 Pa), and the volume of the tank is 15 litres. 5 (a) The oxygen is pumped to his mouth at atmospheric pressure (1.0 x 10 Pa). What is the maximum volume of oxygen available to the astronaut at this pressure? (b) What assumptions are made about the gas that allow us to perform this calculation? (c) In reality, the astronaut would find that he had less oxygen available to him than calculated in part (a). Why would this be the case? (Problem solving!)FORMAL HOMEWORK EXERCISE Radiation & Matter 4. Draw the electric field around the following charges. You must show the direction of the field clearly. (a) - + (b) + - 5. What is the definition of a volt? 6. Look at the following diagram. 1.5 C 300 V 100 V (a) What is the potential difference between the two plates? (b) Calculate the work done in moving the charged particle across the electric field. 7. Most vehicle assembly lines use robots...
Attachments:

1 Approved Answer

Do you need an answer to a question different from the above? Ask your question!
(Hide this section if you want to rate later)
Was the final answer of the question wrong?
Were the solution steps not detailed enough?
Was the language and grammar an issue?
Does the question reference wrong data/report or numbers?
Stay Solved :)
Didn't find yours?
Get plagiarism-free solution within 48 hours
Review Please
Help us make our solutions better
Rate this solution on a scale of 1-5 star

Thank you for your feedback.
Related questions, 3–165 a u-tube contains water in the right arm, and....
3-165Solution A U-tube that...
I attached a picture for my home work My homework contain 5 problems in Mechanics The text book which we use is (Theoretical Mechanics pf particles and continua- Fetter and Walecka)
3–139 balloons are often filled with helium gas because it questions - mechanical eng - fluid mechanics - class homework question - set 139 problem 3-139 questions - mechanical eng - fluid mechanics - class homework question - set 139 problem..., 944 quantum mechanics -homework #3. due: friday, october 17, 2008. 1. this problem is about oscillator strengths in the hydrogen atom (merzbacher chapter 19, exercise 19., hi, i have homework, they are 3 small questions from principles of quantum mechanics by shankar. i hope you answer the homework. see the attached file please, phys851 quantum mechanics i, fall 2009 homework assignment 3: fundamentals of quantum mechanics 1. [10pts] the trace of an operator is defined as tr{a} = pmhm|a|mi, where {|mi} is a suitable basis set., recent questions in management - others, this project requires you to determine and manage a project scope on two separate occasions, question 2 what is a commonly held and persistent assumption about nursing link this back to a stereotype and consider how this assumption/stereotype has impacted your perceptions of the profession. (gender stereotype) in discussing this question:..., introduction: one of the most important aspects of technical writing involves your ability to representyourself to others. the need for such communication skills typically reveals itself first in the effort tosecure gainful employment; resumes and..., xyz breweries ltd has two bottling plants, one located at g , other located at j. each plant produced three drinks whisky, beer and brandy names a, b and c respectively. the number of bottles produce per day are as follows whisky 1500, 11500, beer..., one page report and 7 slides needed without speaker notes but with references question 3: vision statement: indigenous child welfare in this task, you should imagine yourself as a worker in an agency that focusses on work with children in care...., with the help of the following data of a company draw up balance sheet. inventory turnover ………………………………. 6 capital turnover (cost of goods sold)…………….. 2 fixed assets turnover (cost of goods sold)………. 4 gross profit ratio ……………………………….. 20%..., student assessment task 1 bsbpmg530 manage project scope bsb50820 diploma of project management assessment task 1: knowledge questions information for students knowledge questions are designed to help you demonstrate the knowledge which you have..., questions: 1. discuss the wisdoms that philosophers may seek in relation to human life and universe consider the differences and similarities of these wisdoms among philosophers from metaphysical, epistemological, axiological and ethical point of..., according to lisa quast’s “how to manage your boss”, discuss a way how you have managed your boss not previously discussed see article. http://www.forbes.com/sites/lisaquast/2013/04/29/how-to-manage-your-boss, plagiarism checker.
Submit your documents and get free Plagiarism report
Stuck with a Question?
Your solution is just a click away! Get it Now
Couldn't Find What You Were Looking For ?
Get it solved from our top experts within 48hrs!
Good News! We have found the answer to this question!

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

8.E: Chemical Bonding Basics (Exercises)
- Last updated
- Save as PDF
- Page ID 43486
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
8.1: CHEMICAL BONDS, LEWIS SYMBOLS AND THE OCTET RULE
Conceptual problems.
- The Lewis electron system is a simplified approach for understanding bonding in covalent and ionic compounds. Why do chemists still find it useful?
- Is a Lewis dot symbol an exact representation of the valence electrons in an atom or ion? Explain your answer.
- How can the Lewis electron dot system help to predict the stoichiometry of a compound and its chemical and physical properties?
- How is a Lewis dot symbol consistent with the quantum mechanical model of the atom? How is it different?
Conceptual Answer
8.2: ionic bonding.
Describe the differences in behavior between NaOH and CH 3 OH in aqueous solution. Which solution would be a better conductor of electricity? Explain your reasoning.
What is the relationship between the strength of the electrostatic attraction between oppositely charged ions and the distance between the ions? How does the strength of the electrostatic interactions change as the size of the ions increases?
Which will result in the release of more energy: the interaction of a gaseous sodium ion with a gaseous oxide ion or the interaction of a gaseous sodium ion with a gaseous bromide ion? Why?
Which will result in the release of more energy: the interaction of a gaseous chloride ion with a gaseous sodium ion or a gaseous potassium ion? Explain your answer.
What are the predominant interactions when oppositely charged ions are
- at internuclear distances close to r 0 ?
- very close together (at a distance that is less than the sum of the ionic radii)?
Several factors contribute to the stability of ionic compounds. Describe one type of interaction that destabilizes ionic compounds. Describe the interactions that stabilize ionic compounds.
What is the relationship between the electrostatic attractive energy between charged particles and the distance between the particles?
The interaction of a sodium ion and an oxide ion. The electrostatic attraction energy between ions of opposite charge is directly proportional to the charge on each ion ( Q 1 and Q 2 in Equation 9.1). Thus, more energy is released as the charge on the ions increases (assuming the internuclear distance does not increase substantially). A sodium ion has a +1 charge; an oxide ion, a −2 charge; and a bromide ion, a −1 charge. For the interaction of a sodium ion with an oxide ion, Q 1 = +1 and Q 2 = −2, whereas for the interaction of a sodium ion with a bromide ion, Q 1 = +1 and Q 2 = −1. The larger value of Q 1 × Q 2 for the sodium ion–oxide ion interaction means it will release more energy.
Numerical Problems
How does the energy of the electrostatic interaction between ions with charges +1 and −1 compare to the interaction between ions with charges +3 and −1 if the distance between the ions is the same in both cases? How does this compare with the magnitude of the interaction between ions with +3 and −3 charges?
How many grams of gaseous MgCl 2 are needed to give the same electrostatic attractive energy as 0.5 mol of gaseous LiCl? The ionic radii are Li + = 76 pm, Mg +2 = 72 pm, and Cl − = 181 pm.
Sketch a diagram showing the relationship between potential energy and internuclear distance (from r = ∞ to r = 0) for the interaction of a bromide ion and a potassium ion to form gaseous KBr. Explain why the energy of the system increases as the distance between the ions decreases from r = r 0 to r = 0.
Calculate the magnitude of the electrostatic attractive energy ( E , in kilojoules) for 85.0 g of gaseous SrS ion pairs. The observed internuclear distance in the gas phase is 244.05 pm.
What is the electrostatic attractive energy ( E , in kilojoules) for 130 g of gaseous HgI 2 ? The internuclear distance is 255.3 pm.
Numerical Answers
According to Equation 9.1, in the first case Q 1 Q 2 = (+1)(−1) = −1; in the second case, Q 1 Q 2 = (+3)(−1) = −3. Thus, E will be three times larger for the +3/−1 ions. For +3/−3 ions, Q 1 Q 2 = (+3)(−3) = −9, so E will be nine times larger than for the +1/−1 ions.
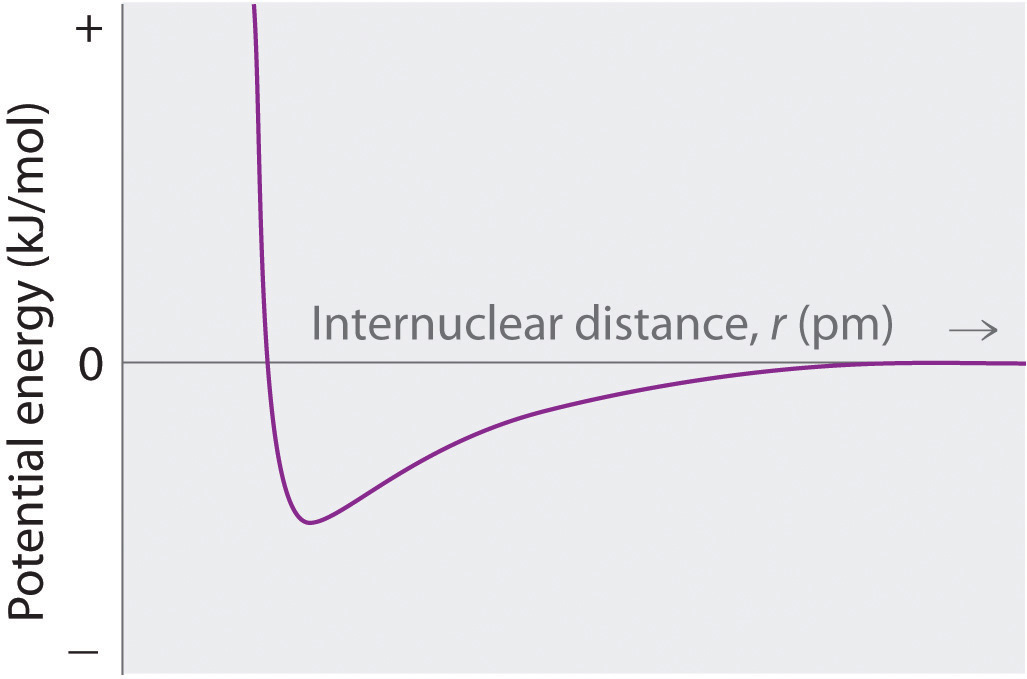
At r < r 0 , the energy of the system increases due to electron–electron repulsions between the overlapping electron distributions on adjacent ions. At very short internuclear distances, electrostatic repulsions between adjacent nuclei also become important.
8.3: COVALENT BONDING
- Which would you expect to be stronger—an S–S bond or an Se–Se bond? Why?
- Which element—nitrogen, phosphorus, or arsenic—will form the strongest multiple bond with oxygen? Why?
- Why do multiple bonds between oxygen and period 3 elements tend to be unusually strong?
- What can bond energies tell you about reactivity?
- Bond energies are typically reported as average values for a range of bonds in a molecule rather than as specific values for a single bond? Why?
- If the bonds in the products are weaker than those in the reactants, is a reaction exothermic or endothermic? Explain your answer.
- A student presumed that because heat was required to initiate a particular reaction, the reaction product would be stable. Instead, the product exploded. What information might have allowed the student to predict this outcome?
What is the bond order about the central atom(s) of hydrazine (N 2 H 4 ), nitrogen, and diimide (N 2 H 2 )? Draw Lewis electron structures for each compound and then arrange these compounds in order of increasing N–N bond distance. Which of these compounds would you expect to have the largest N–N bond energy? Explain your answer.
What is the carbon–carbon bond order in ethylene (C 2 H 4 ), BrH 2 CCH 2 Br, and FCCH? Arrange the compounds in order of increasing C–C bond distance. Which would you expect to have the largest C–C bond energy? Why?
From each pair of elements, select the one with the greater bond strength? Explain your choice in each case.
- P–P, Sb–Sb
- Cl–Cl, I–I
- O–O, Se–Se
- S–S, Cl–Cl
- Al–Cl, B–Cl
- Te–Te, S–S
- C–H, Ge–H
- Si–Si, P–P
- Cl–Cl, F–F
- Ga–H, Al–H
Approximately how much energy per mole is required to completely dissociate acetone [(CH 3 ) 2 CO] and urea [(NH 2 ) 2 CO] into their constituent atoms?
Approximately how much energy per mole is required to completely dissociate ethanol, formaldehyde, and hydrazine into their constituent atoms?
Is the reaction of diimine (N 2 H 2 ) with oxygen to produce nitrogen and water exothermic or endothermic? Quantify your answer.
Numerical Answer
N 2 H 4 , bond order 1; N 2 H 2 , bond order 2; N 2 , bond order 3; N–N bond distance: N 2 < N 2 H 2 < N 2 H 4 ; Largest bond energy: N 2 ; Highest bond order correlates with strongest and shortest bond.
8.4: BOND POLARITY AND ELECRONEGATIVITY
Why do ionic compounds such as KI exhibit substantially less than 100% ionic character in the gas phase?
Of the compounds LiI and LiF, which would you expect to behave more like a classical ionic compound? Which would have the greater dipole moment in the gas phase? Explain your answers.
Predict whether each compound is purely covalent, purely ionic, or polar covalent.
Based on relative electronegativities, classify the bonding in each compound as ionic, covalent, or polar covalent. Indicate the direction of the bond dipole for each polar covalent bond.
- the C=O bond in acetone
- the S–S bond in CH 3 CH 2 SSCH 2 CH 3
- the C–Cl bond in CH 2 Cl 2
- the O–H bond in CH 3 OH
- PtCl 4 2 −
Classify each species as having 0%–40% ionic character, 40%–60% ionic character, or 60%–100% ionic character based on the type of bonding you would expect. Justify your reasoning.
If the bond distance in HCl (dipole moment = 1.109 D) were double the actual value of 127.46 pm, what would be the effect on the charge localized on each atom? What would be the percent negative charge on Cl? At the actual bond distance, how would doubling the charge on each atom affect the dipole moment? Would this represent more ionic or covalent character?
Calculate the percent ionic character of HF (dipole moment = 1.826 D) if the H–F bond distance is 92 pm.
Calculate the percent ionic character of CO (dipole moment = 0.110 D) if the C–O distance is 113 pm.
Calculate the percent ionic character of PbS and PbO in the gas phase, given the following information: for PbS, r = 228.69 pm and µ = 3.59 D; for PbO, r = 192.18 pm and µ = 4.64 D. Would you classify these compounds as having covalent or polar covalent bonds in the solid state?
8.5: DRAWING LEWIS STRUCTURES
Compare and contrast covalent and ionic compounds with regard to
- volatility.
- melting point.
- electrical conductivity.
- physical appearance.
What are the similarities between plots of the overall energy versus internuclear distance for an ionic compound and a covalent compound? Why are the plots so similar?
Which atom do you expect to be the central atom in each of the following species?
- SO 4 2 −
Which atom is the central atom in each of the following species?
What is the relationship between the number of bonds typically formed by the period 2 elements in groups 14, 15, and 16 and their Lewis electron structures?
Although formal charges do not represent actual charges on atoms in molecules or ions, they are still useful. Why?
Give the electron configuration and the Lewis dot symbol for the following. How many more electrons can each atom accommodate?
Based on Lewis dot symbols, predict the preferred oxidation state of Be, F, B, and Cs.
Based on Lewis dot symbols, predict the preferred oxidation state of Br, Rb, O, Si, and Sr.
Based on Lewis dot symbols, predict how many bonds gallium, silicon, and selenium will form in their neutral compounds.
Determine the total number of valence electrons in the following.
- NO 3 −
Draw Lewis electron structures for the following.
- AlCl 4 −
- SO 3 2 −
- S 2 2 −
Draw Lewis electron structures for CO 2 , NO 2 − , SO 2 , and NO 2 + . From your diagram, predict which pair(s) of compounds have similar electronic structures.
Write Lewis dot symbols for each pair of elements. For a reaction between each pair of elements, predict which element is the oxidant, which element is the reductant, and the final stoichiometry of the compound formed.
Use Lewis dot symbols to predict whether ICl and NO 4 − are chemically reasonable formulas.
Draw a plausible Lewis electron structure for a compound with the molecular formula Cl 3 PO.
Draw a plausible Lewis electron structure for a compound with the molecular formula CH 4 O.
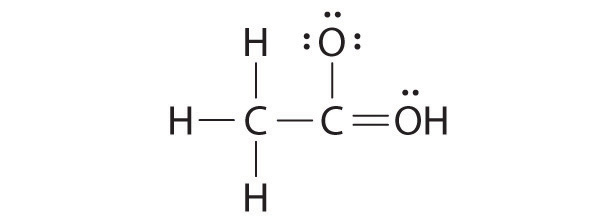
While reviewing her notes, a student noticed that she had drawn the following structure in her notebook for acetic acid:
Why is this structure not feasible? Draw an acceptable Lewis structure for acetic acid. Show the formal charges of all nonhydrogen atoms in both the correct and incorrect structures.
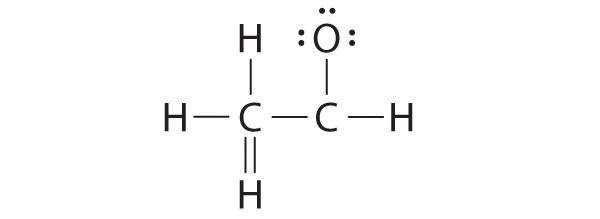
A student proposed the following Lewis structure shown for acetaldehyde.
Why is this structure not feasible? Draw an acceptable Lewis structure for acetaldehyde. Show the formal charges of all nonhydrogen atoms in both the correct and incorrect structures.
Draw the most likely structure for HCN based on formal charges, showing the formal charge on each atom in your structure. Does this compound have any plausible resonance structures? If so, draw one.
Draw the most plausible Lewis structure for NO 3 − . Does this ion have any other resonance structures? Draw at least one other Lewis structure for the nitrate ion that is not plausible based on formal charges.
At least two Lewis structures can be drawn for BCl 3 . Using arguments based on formal charges, explain why the most likely structure is the one with three B–Cl single bonds.
Using arguments based on formal charges, explain why the most feasible Lewis structure for SO 4 2 − has two sulfur–oxygen double bonds.
At least two distinct Lewis structures can be drawn for N 3 − . Use arguments based on formal charges to explain why the most likely structure contains a nitrogen–nitrogen double bond.
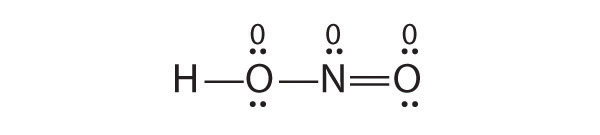
Is H–O–N=O a reasonable structure for the compound HNO 2 ? Justify your answer using Lewis electron dot structures.
Is H–O=C–H a reasonable structure for a compound with the formula CH 2 O? Use Lewis electron dot structures to justify your answer.
Explain why the following Lewis structure for SO 3 2 − is or is not reasonable.
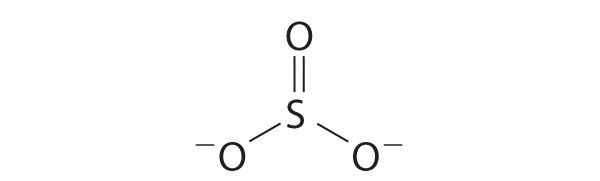
[Ar]4 s 2 3 d 10 4 p 4
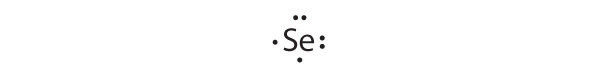
Selenium can accommodate two more electrons, giving the Se 2 − ion.
[Ar]4 s 2 3 d 10 4 p 6
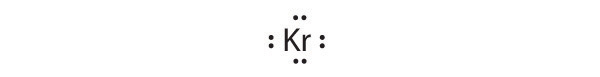
Krypton has a closed shell electron configuration, so it cannot accommodate any additional electrons.
1 s 2 2 s 1
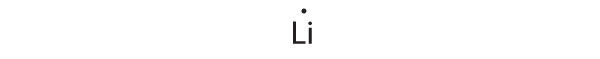
Lithium can accommodate one additional electron in its 2 s orbital, giving the Li − ion.
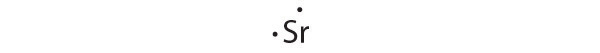
Strontium has a filled 5 s subshell, and additional electrons would have to be placed in an orbital with a higher energy. Thus strontium has no tendency to accept an additional electron.

Hydrogen can accommodate one additional electron in its 1 s orbital, giving the H − ion.
Be 2 + , F − , B 3+ , Cs +
K is the reductant; S is the oxidant. The final stoichiometry is K 2 S.
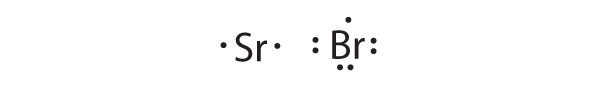
Sr is the reductant; Br is the oxidant. The final stoichiometry is SrBr 2 .
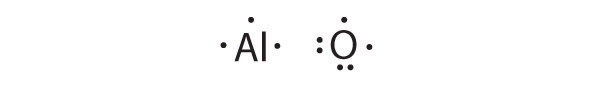
Al is the reductant; O is the oxidant. The final stoichiometry is Al 2 O 3 .

Mg is the reductant; Cl is the oxidant. The final stoichiometry is MgCl 2 .
The only structure that gives both oxygen and carbon an octet of electrons is the following:
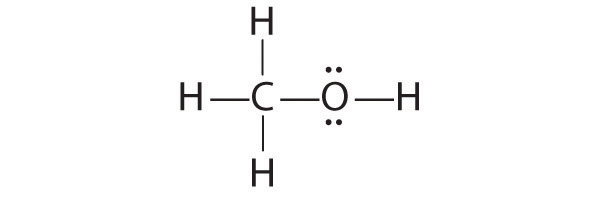
The student’s proposed structure has two flaws: the hydrogen atom with the double bond has four valence electrons (H can only accommodate two electrons), and the carbon bound to oxygen only has six valence electrons (it should have an octet). An acceptable Lewis structure is
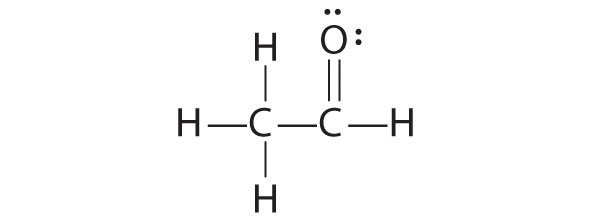
The formal charges on the correct and incorrect structures are as follows:

The most plausible Lewis structure for NO 3 − is:
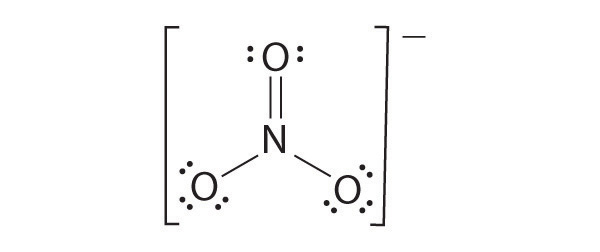
There are three equivalent resonance structures for nitrate (only one is shown), in which nitrogen is doubly bonded to one of the three oxygens. In each resonance structure, the formal charge of N is +1; for each singly bonded O, it is −1; and for the doubly bonded oxygen, it is 0.
The following is an example of a Lewis structure that is not plausible:
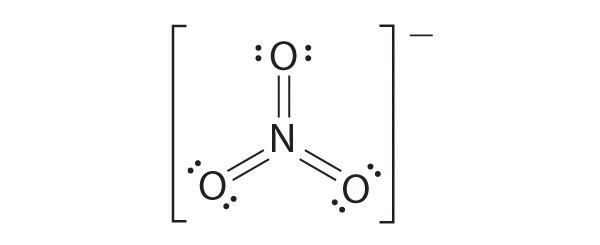
This structure nitrogen has six bonds (nitrogen can form only four bonds) and a formal charge of –1.
With four S–O single bonds, each oxygen in SO 4 2 − has a formal charge of −1, and the central sulfur has a formal charge of +2. With two S=O double bonds, only two oxygens have a formal charge of –1, and sulfur has a formal charge of zero. Lewis structures that minimize formal charges tend to be lowest in energy, making the Lewis structure with two S=O double bonds the most probable.
8.6: RESONANCE STRUCTURES
- Why are resonance structures important?
- In what types of compounds are resonance structures particularly common?
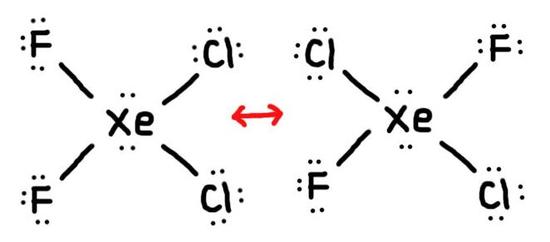
- True or False, The picture below is a resonance structure?
- HSO 4 −
- HSO 3 −
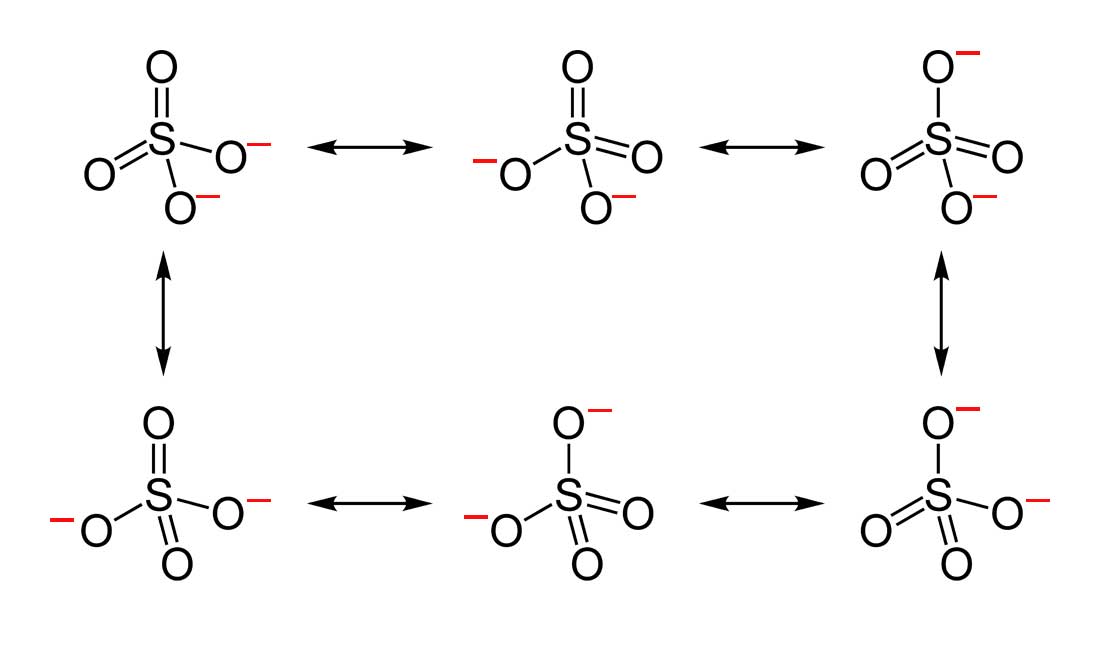
- Draw the Lewis Dot Structure for SO 4 2 - and all possible resonance structures. Which of the following resonance structure is not favored among the Lewis Structures? Explain why. Assign Formal Charges.
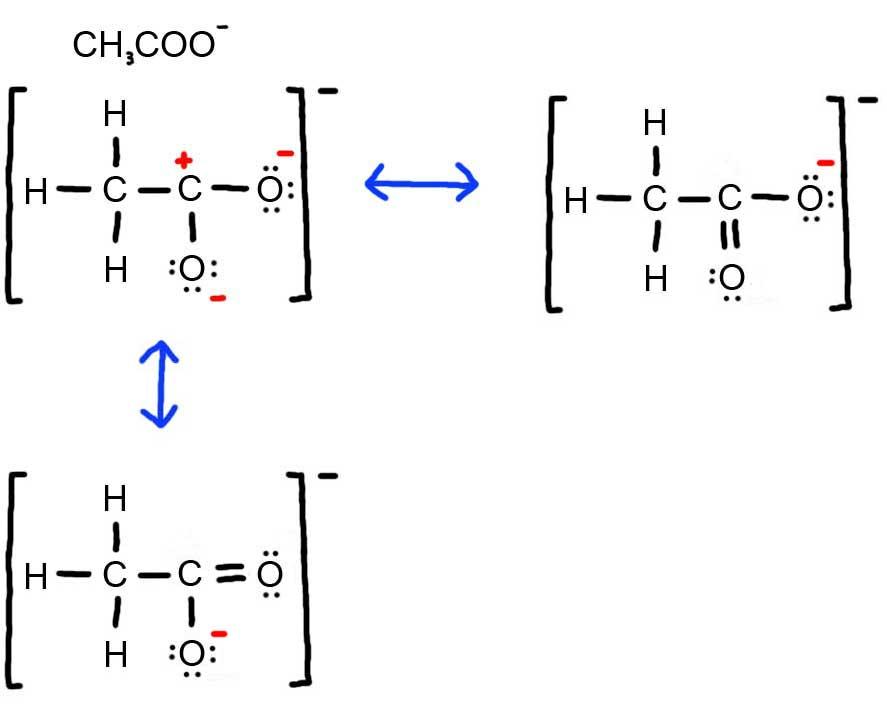
- Draw the Lewis Dot Structure for CH 3 COO - and all possible resonance structures. Assign Formal Charges. Choose the most favorable Lewis Structure.
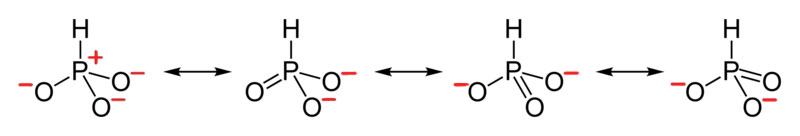
- Draw the Lewis Dot Structure for H PO 3 2 - and all possible resonance structures. Assign Formal Charges.
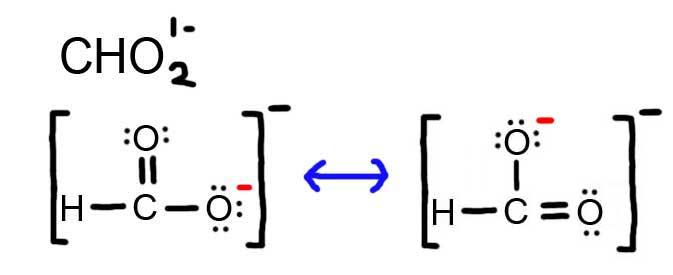
- Draw the Lewis Dot Structure for CHO 2 1 - and all possible resonance structures. Assign Formal Charges.
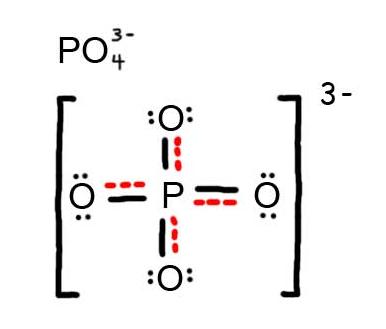
- Draw the Resonance Hybrid Structure for P O 4 3 - .
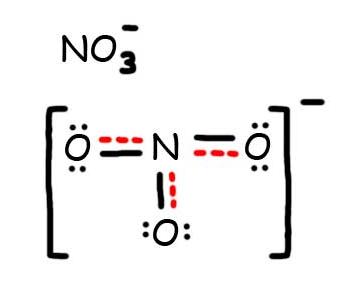
- Draw the Resonance Hybrid Structure for N O 3 - .
1. False, because the electrons were not moved around, only the atoms (this violates the Resonance Structure Rules).
3. Below are the all Lewis dot structure with formal charges (in red) for Sulfate ( SO 4 2 - ). There isn't a most favorable resonance of the Sulfate ion because they are all identical in charge and there is no change in Electronegativity between the Oxygen atoms.
4. Below is the resonance for CH 3 COO - , formal charges are displayed in red. The Lewis Structure with the most formal charges is not desirable, because we want the Lewis Structure with the least formal charge.
5. The resonance for HPO 3 2 - , and the formal charges (in red).
6. The resonance for CHO 2 1 - , and the formal charges (in red).
7. The resonance hybrid for PO 4 3 - , hybrid bonds are in red.
8. The resonance hybrid for NO 3 - , hybrid bonds are in red.
8.7: EXCEPTIONS TO THE OCTET RULE
- What regions of the periodic table contain elements that frequently form molecules with an odd number of electrons? Explain your answer.
- How can atoms expand their valence shell? What is the relationship between an expanded valence shell and the stability of an ion or a molecule?
- What elements are known to form compounds with less than an octet of electrons? Why do electron-deficient compounds form?
- List three elements that form compounds that do not obey the octet rule. Describe the factors that are responsible for the stability of these compounds.
What is the major weakness of the Lewis system in predicting the electron structures of PCl 6 − and other species containing atoms from period 3 and beyond?
The compound aluminum trichloride consists of Al 2 Cl 6 molecules with the following structure (lone pairs of electrons removed for clarity):
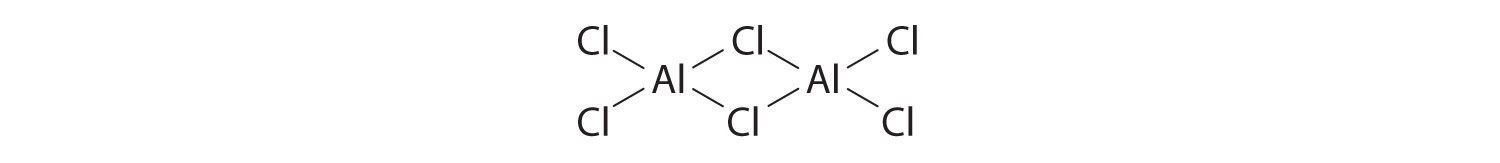
Does this structure satisfy the octet rule? What is the formal charge on each atom? Given the chemical similarity between aluminum and boron, what is a plausible explanation for the fact that aluminum trichloride forms a dimeric structure rather than the monomeric trigonal planar structure of BCl 3 ?
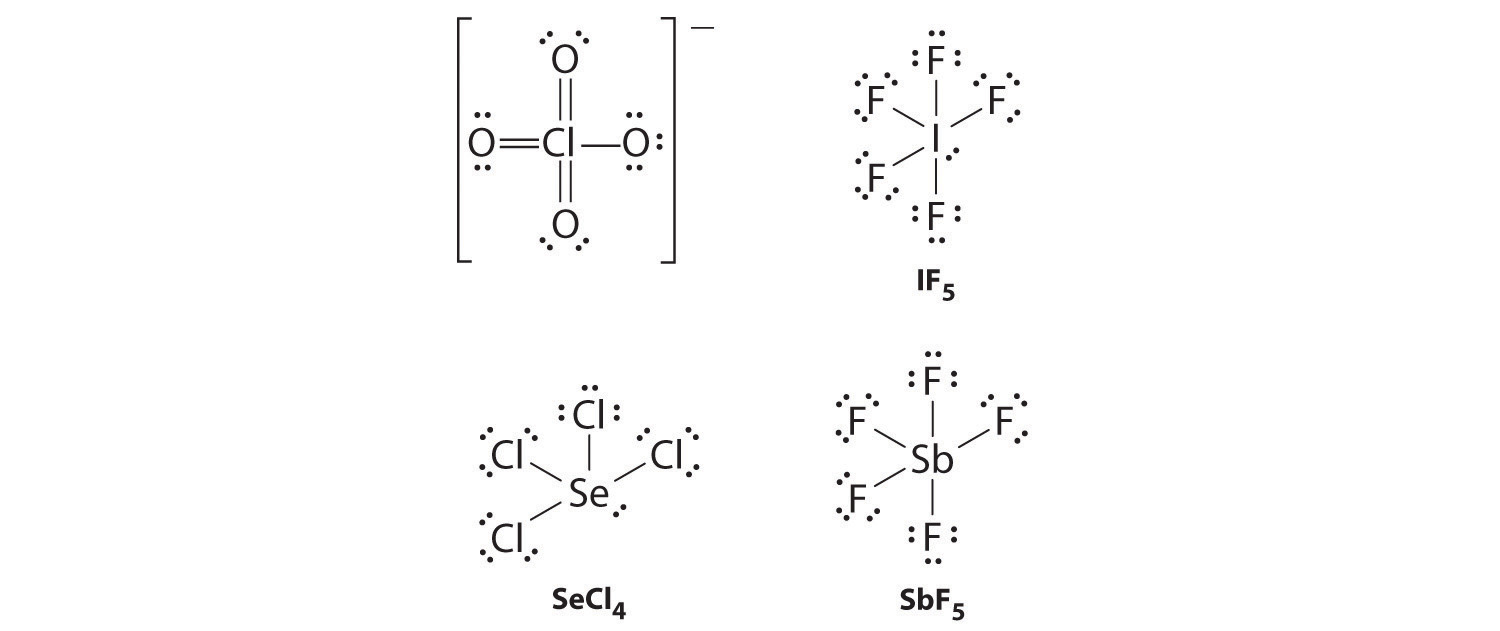
Draw Lewis electron structures for ClO 4 − , IF 5 , SeCl 4 , and SbF 5 .
Draw Lewis electron structures for ICl 3 , Cl 3 PO, Cl 2 SO, and AsF 6 − .
Draw plausible Lewis structures for the phosphate ion, including resonance structures. What is the formal charge on each atom in your structures?
Draw an acceptable Lewis structure for PCl 5 , a compound used in manufacturing a form of cellulose. What is the formal charge of the central atom? What is the oxidation number of the central atom?
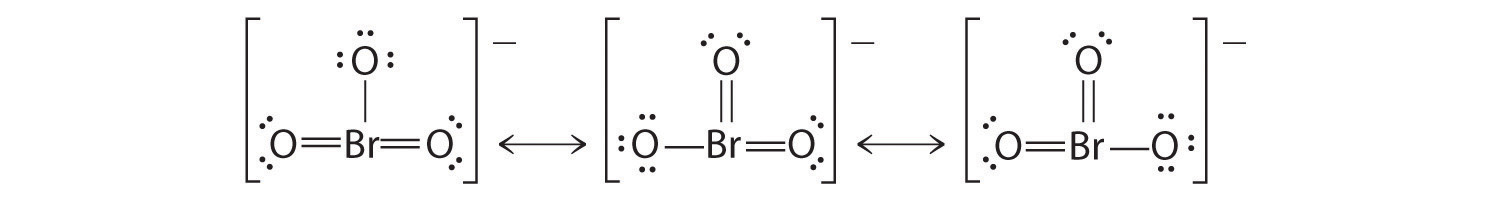
Using Lewis structures, draw all of the resonance structures for the BrO 3 − ion.
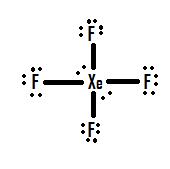
Draw an acceptable Lewis structure for xenon trioxide (XeO 3 ), including all resonance structures.
ClO 4 − (one of four equivalent resonance structures)
The formal charge on phosphorus is 0, while three oxygen atoms have a formal charge of −1 and one has a formal charge of zero.
Practice Problems
- Draw the Lewis structure for the molecule I 3 - .
- Draw the molecule ClF 3 .
- The central atom for an expanded octet must have an atomic number larger than what?
- Draw the Lewis structure for the molecule NO 2 .
- Which Lewis structure is more likely?
Practice Answers
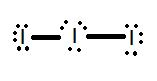
3. 10 (Sodium and higher)
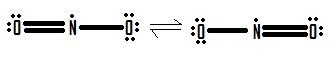
8.8: STRENGTHS OF COVALENT BONDS

IMAGES
VIDEO
COMMENTS
Mechanics & Properties of Matter Higher Homework for Tuesday 28th September 1. A boy using a force of 250 N pulls a sledge across the snow as shown in the diagram below. ... FORMAL HOMEWORK EXERCISE Mechanics & Properties of Matter 4. A train made up of 3 carriages is pulled along a level track by a force of 16 500 N. Each of the carriages has ...
Strathaven Academy 19 August, 2010 Formal Homework Exercise 9 of 20 FORMAL HOMEWORK EXERCISE Mechanics & Properties of Matter 5. An astronaut on a spacewalk has an oxygen tank strapped to his suit. The oxygen in it is pressurised to 5 atmospheres (5.0 x 105 Pa), and the volume of the tank is 15 litres.
Strathaven Academy 4 April, 2009 Formal Homework Answers 3 of 20 HOMEWORK MARKING SCHEME Mechanics & Properties of Matter Homework 3 - Vectors 1. (a) A vector has both a magnitude and a direction, and a scalar has a magnitude only. (1) (b) VECTOR: displacement, velocity, acceleration, force, weight, momentum.
Keep a note of your Higher Physics formal homework exercises on this sheet. The Due Date should be filled in whenever you are told it, to ensure there are no misunderstandings about deadlines. Once the homework has been returned, fill in ... Mechanics & Properties of Matter. Homework 4 - Equations Of Motion . 1. A workman on the scaffolding ...
Properties of matter (Course Intro) Google Classroom. Microsoft Teams. About. Everest stands at about 9 KM. But, Mountains on earth can never be taller than about 10 KM. But why? Why is steel more elastic than elastic bands, even though these bands stretch so much while steel hardly does! When you gently place it, a paper clip can float on water.
1.E: Matter and Measurement (Exercises) Page ID. These are homework exercises to accompany the Textmap created for "Chemistry: The Central Science" by Brown et al. Complementary General Chemistry question banks can be found for other Textmaps and can be accessed here. In addition to these publicly available questions, access to private problems ...
Learn about the properties of matter, such as mass, volume, density, and solubility, with free interactive resources and activities from PBS LearningMedia. Explore how matter can change from one state to another, and how physical and chemical properties can be observed and measured.
The solubility of a gas in a liquid is the greatest at low temperature and high pressure. This page titled 7.E: Solids, Liquids, and Gases (Exercises) is shared under a CK-12 license and was authored, remixed, and/or curated by CK-12 Foundation. These are homework exercises to accompany Chapter 7 of the University of Kentucky's LibreText for ...
Strathaven Academy 18 November, 2008 Formal Homework Exercise 7 of 20 FORMAL HOMEWORK EXERCISE Mechanics & Properties of Matter Homework 7 - Pressure And Density 1. The stools in the physics rooms have four round feet, each with a surface area of approximately 1 cm2. The physics teachers complain that when people swing onto
Physics and Mechanics q Physics deals with the nature and properties of matter and energy. Common language is math (Co-Reqs: Math 111 or Math 132). Physics is based on experimental observations and quantitative measurements. q The study of physics can be divided into six main areas: n Classical mechanics - Physics I (Phys. 111)
This topic is part of the study of statics in mechanics. In such a case, our initial reaction might be to claim that nothing happens, but this overlooks the fact that the rod is not a simple point
Mechanical properties of matter. stress, strain, Young modulus. force-extension graphs, energy stored. For many students, this topic will be the first time in physics they have been asked to explicitly link microscale structure (molecular bonds) with observed behaviour (stiffness and other characteristics). Their familiarity with the language ...
Given 15.00 g of each element, calculate the volume of each and then arrange the elements in order of increasing volume. The numbers in parentheses are densities. gold (19.32 g/cm3) lead (11.34 g/cm3) iron (7.87 g/cm3) sulfur (2.07 g/cm3) A silver bar has dimensions of 10.00 cm × 4.00 cm × 1.50 cm, and the density of silver is 10.49 g/cm3.
Physics: Mechanics and Properties of Matter (H) - Student Material 2 Momentum and impulse 1. State that momentum is the product of mass and velocity. 2. State that the law of conservation of linear momentum can be applied to the interaction of two objects moving in one dimension, in the absence of net external forces. 3.
2.1 Different Form of Energy used in Mechanics 2.1.1 Kinetic energy 2.1.2 Potential energy 2.2 The work-energy Theorem 2.3 Work Done by a Uniform Force 2.4 Non conservative forces 2.5 Energy Diagrams 2.5.1 Energy Diagram of Bounded Motion 2.5.2 Energy Diagram of Unbounded Motion 2.6 Stability and Unstability in One Dimension
1.2: Properties of Matter; 1.3: Classification of Matter Matter is anything that occupies space and has mass. The basic building block of matter is the atom, the smallest unit of an element that can enter into combinations with atoms of the same or other elements. In many substances, atoms are combined into molecules.
An understanding of how objects becomes charged begins with an understanding of the structure of the atom. The atom consists of uncharged neutrons and positively-charged protons densely packed into the center of the atom - known as the nucleus. Surrounding the nucleus are negatively-charged electrons that are located in regions of space known as electron shells.
Some properties of matter, such as solubility, describe how matter physically changes, while others, such as mass or volume, simply provide the matter's measurements. Watch this video to find out more about the different ways of describing and measuring matter.
Mechanics & Properties of Matter Higher Homework for Tuesday 20th September 1. A boy using a force of 250 N pulls a sledge across the snow as shown in the diagram below. ... FORMAL HOMEWORK EXERCISE Mechanics & Properties of Matter 4. A train made up of 3 carriages is pulled along a level track by a force of 16 500 N. Each of the carriages has ...
FORMAL HOMEWORK EXERCISE Mechanics & Properties of Matter Homework - Forces 1. A train made up of 3 carriages is pulled along a level track by a force of 16 500 N. Each of the carriages has a mass of 8 000 kg, and each experiences 1500 N of resistive forces. (a) Calculate the acceleration of the train. (2) (b) Work out the tension in link B. (2) 2.
FORMAL HOMEWORK EXERCISE Mechanics & Properties of Matter Attempt all 7 questions Homework is due by Monday 15 December 1. A car has its tyres inflated to a pressure of 240 kPa on a day when the temperature is 5 °C. The car is then driven for several hours, and the temperature of the tyres is found to have risen to 35 °C.
Thus, E will be three times larger for the +3/−1 ions. For +3/−3 ions, Q1Q2 = (+3) (−3) = −9, so E will be nine times larger than for the +1/−1 ions. At r < r0, the energy of the system increases due to electron-electron repulsions between the overlapping electron distributions on adjacent ions.
Strathaven Academy 19 August, 2010 Formal Homework Exercise 8 of 20 FORMAL HOMEWORK EXERCISE Mechanics & Properties of Matter Homework: Pressure, Density & Gas Laws 1. The stools in the physics rooms have four round feet, each with a surface area of approximately 1 cm2. The physics teachers complain that when people swing onto