If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.

High school physics
Course: high school physics > unit 5.
- Law of conservation of energy
- LOL diagrams
- Conservation of energy: Predict changes in energy
- Conservation of energy: Numerical calculations
Conservation of energy review
How to write the conservation of energy equation
- Draw a picture of the scenario, list your known information, and identify your system. Don’t forget that potential energy and work done by friction must include two objects.
- Decide what the initial and final locations will be for analyzing energy conservation by including our desired unknown in one of the locations and all the known information in the other location. Label the kinetic and potential energies at these two points.
- Designate the lower of the two positions as the zero height location. This eliminates the potential energy term for this location and simplifies our conservation of energy equation.
- If there are no nonconservative forces like friction, then use the conservation of mechanical energy:
- Cancel out any of the energy terms that are zero to simplify your equation. For example, if the system has no motion at the final or initial positions, then remove the kinetic energy terms from the equation.
Common mistakes and misconceptions
- The conservation of energy equation only compares a system’s energy for the final and initial points in time. There may be different combinations of energy between these two points, but the equation we use only considers the final and initial energies.
- People mistakenly think energy is constant for an object. The total energy of the universe is constant, but energy can be transferred between systems that we define in the universe. If one system gains energy, some other system must have lost energy to conserve the total energy in the universe.
Want to join the conversation?
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page
7.6 Conservation of Energy
Learning objectives.
By the end of this section, you will be able to:
- Explain the law of the conservation of energy.
- Describe some of the many forms of energy.
- Define efficiency of an energy conversion process as the fraction left as useful energy or work, rather than being transformed, for example, into thermal energy.
Law of Conservation of Energy
Energy, as we have noted, is conserved, making it one of the most important physical quantities in nature. The law of conservation of energy can be stated as follows:
Total energy is constant in any process. It may change in form or be transferred from one system to another, but the total remains the same.
We have explored some forms of energy and some ways it can be transferred from one system to another. This exploration led to the definition of two major types of energy—mechanical energy KE + PE KE + PE and energy transferred via work done by nonconservative forces ( W nc ) ( W nc ) . But energy takes many other forms, manifesting itself in many different ways, and we need to be able to deal with all of these before we can write an equation for the above general statement of the conservation of energy.
Other Forms of Energy than Mechanical Energy
At this point, we deal with all other forms of energy by lumping them into a single group called other energy ( OE OE ). Then we can state the conservation of energy in equation form as
All types of energy and work can be included in this very general statement of conservation of energy. Kinetic energy is KE KE , work done by a conservative force is represented by PE PE , work done by nonconservative forces is W nc W nc , and all other energies are included as OE OE . This equation applies to all previous examples; in those situations OE OE was constant, and so it subtracted out and was not directly considered.
Making Connections: Usefulness of the Energy Conservation Principle
The fact that energy is conserved and has many forms makes it very important. You will find that energy is discussed in many contexts, because it is involved in all processes. It will also become apparent that many situations are best understood in terms of energy and that problems are often most easily conceptualized and solved by considering energy.
When does OE OE play a role? One example occurs when a person eats. Food is oxidized with the release of carbon dioxide, water, and energy. Some of this chemical energy is converted to kinetic energy when the person moves, to potential energy when the person changes altitude, and to thermal energy (another form of OE OE ).
Some of the Many Forms of Energy
What are some other forms of energy? You can probably name a number of forms of energy not yet discussed. Many of these will be covered in later chapters, but let us detail a few here. Electrical energy is a common form that is converted to many other forms and does work in a wide range of practical situations. Fuels, such as gasoline and food, carry chemical energy that can be transferred to a system through oxidation. Chemical fuel can also produce electrical energy, such as in batteries. Batteries can in turn produce light, which is a very pure form of energy. Most energy sources on Earth are in fact stored energy from the energy we receive from the Sun. We sometimes refer to this as radiant energy , or electromagnetic radiation, which includes visible light, infrared, and ultraviolet radiation. Nuclear energy comes from processes that convert measurable amounts of mass into energy. Nuclear energy is transformed into the energy of sunlight, into electrical energy in power plants, and into the energy of the heat transfer and blast in weapons. Atoms and molecules inside all objects are in random motion. This internal mechanical energy from the random motions is called thermal energy , because it is related to the temperature of the object. These and all other forms of energy can be converted into one another and can do work.
Table 7.1 gives the amount of energy stored, used, or released from various objects and in various phenomena. The range of energies and the variety of types and situations is impressive.
Problem-Solving Strategies for Energy
You will find the following problem-solving strategies useful whenever you deal with energy. The strategies help in organizing and reinforcing energy concepts. In fact, they are used in the examples presented in this chapter. The familiar general problem-solving strategies presented earlier—involving identifying physical principles, knowns, and unknowns, checking units, and so on—continue to be relevant here.
Step 1. Determine the system of interest and identify what information is given and what quantity is to be calculated. A sketch will help.
Step 2. Examine all the forces involved and determine whether you know or are given the potential energy from the work done by the forces. Then use step 3 or step 4.
Step 3. If you know the potential energies for the forces that enter into the problem, then forces are all conservative, and you can apply conservation of mechanical energy simply in terms of potential and kinetic energy. The equation expressing conservation of energy is
Step 4. If you know the potential energy for only some of the forces, possibly because some of them are nonconservative and do not have a potential energy, or if there are other energies that are not easily treated in terms of force and work, then the conservation of energy law in its most general form must be used.
In most problems, one or more of the terms is zero, simplifying its solution. Do not calculate W c W c , the work done by conservative forces; it is already incorporated in the PE PE terms.
Step 5. You have already identified the types of work and energy involved (in step 2). Before solving for the unknown, eliminate terms wherever possible to simplify the algebra. For example, choose h = 0 h = 0 at either the initial or final point, so that PE g PE g is zero there. Then solve for the unknown in the customary manner.
Step 6. Check the answer to see if it is reasonable . Once you have solved a problem, reexamine the forms of work and energy to see if you have set up the conservation of energy equation correctly. For example, work done against friction should be negative, potential energy at the bottom of a hill should be less than that at the top, and so on. Also check to see that the numerical value obtained is reasonable. For example, the final speed of a skateboarder who coasts down a 3-m-high ramp could reasonably be 20 km/h, but not 80 km/h.
Transformation of Energy
The transformation of energy from one form into others is happening all the time. The chemical energy in food is converted into thermal energy through metabolism; light energy is converted into chemical energy through photosynthesis. In a larger example, the chemical energy contained in coal is converted into thermal energy as it burns to turn water into steam in a boiler. This thermal energy in the steam in turn is converted to mechanical energy as it spins a turbine, which is connected to a generator to produce electrical energy. (In all of these examples, not all of the initial energy is converted into the forms mentioned. This important point is discussed later in this section.)
Another example of energy conversion occurs in a solar cell. Sunlight impinging on a solar cell (see Figure 7.19 ) produces electricity, which in turn can be used to run an electric motor. Energy is converted from the primary source of solar energy into electrical energy and then into mechanical energy.
Even though energy is conserved in an energy conversion process, the output of useful energy or work will be less than the energy input. The efficiency Eff Eff of an energy conversion process is defined as
Table 7.2 lists some efficiencies of mechanical devices and human activities. In a coal-fired power plant, for example, about 40% of the chemical energy in the coal becomes useful electrical energy. The other 60% transforms into other (perhaps less useful) energy forms, such as thermal energy, which is then released to the environment through combustion gases and cooling towers.
PhET Explorations
Masses and springs.
A realistic mass and spring laboratory. Hang masses from springs and adjust the spring stiffness and damping. You can even slow time. Transport the lab to different planets. A chart shows the kinetic, potential, and thermal energies for each spring.
- 1 Representative values
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/college-physics-2e/pages/1-introduction-to-science-and-the-realm-of-physics-physical-quantities-and-units
- Authors: Paul Peter Urone, Roger Hinrichs
- Publisher/website: OpenStax
- Book title: College Physics 2e
- Publication date: Jul 13, 2022
- Location: Houston, Texas
- Book URL: https://openstax.org/books/college-physics-2e/pages/1-introduction-to-science-and-the-realm-of-physics-physical-quantities-and-units
- Section URL: https://openstax.org/books/college-physics-2e/pages/7-6-conservation-of-energy
© Jan 19, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

6.7: Conservation of Energy
- Last updated
- Save as PDF
- Page ID 26528

\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
By the end of this section, you will be able to:
- Explain the law of the conservation of energy.
- Describe some of the many forms of energy.
- Define efficiency of an energy conversion process as the fraction left as useful energy or work, rather than being transformed, for example, into thermal energy.
Energy, as we have noted, is conserved, making it one of the most important physical quantities in nature. The law of conservation of energy can be stated as follows:
We have explored some forms of energy and some ways it can be transferred from one system to another. This exploration led to the definition of two major types of energy—mechanical energy \((KE + PE)\) and energy transferred via work done by nonconservative forces \((W_{nc})\) But energy takes many other forms, manifesting itself in many different ways, and we need to be able to deal with all of these before we can write an equation for the above general statement of the conservation of energy.
Other Forms of Energy than Mechanical Energy
At this point, we deal with all other forms of energy by lumping them into a single group called other energy \((OE)\). Then we can state the conservation of energy in equation form as
\[KE_i + PE_i + W_{nc} + OE_i = KE_f + PE_f + OE_f .\]
All types of energy and work can be included in this very general statement of conservation of energy. Kinetic energy is \(KE\), work done by a conservative force is represented by \(PE\), work done by nonconservative forces is \(W_{nc}\) and all other energies are included as \(OE\). This equation applies to all previous examples; in those situations \(OE\) was constant, and so it subtracted out and was not directly considered.
Usefulness of the Energy Conservation Principle
The fact that energy is conserved and has many forms makes it very important. You will find that energy is discussed in many contexts, because it is involved in all processes. It will also become apparent that many situations are best understood in terms of energy and that problems are often most easily conceptualized and solved by considering energy.
When does \(OE\) play a role? One example occurs when a person eats. Food is oxidized with the release of carbon dioxide, water, and energy. Some of this chemical energy is converted to kinetic energy when the person moves, to potential energy when the person changes altitude, and to thermal energy (another form of \(OE\)).
Some of the Many Forms of Energy
What are some other forms of energy? You can probably name a number of forms of energy not yet discussed. Many of these will be covered in later chapters, but let us detail a few here. Electrical energy is a common form that is converted to many other forms and does work in a wide range of practical situations. Fuels, such as gasoline and food, carry chemical energy that can be transferred to a system through oxidation. Chemical fuel can also produce electrical energy, such as in batteries. Batteries can in turn produce light, which is a very pure form of energy. Most energy sources on Earth are in fact stored energy from the energy we receive from the Sun. We sometimes refer to this as radiant energy , or electromagnetic radiation, which includes visible light, infrared, and ultraviolet radiation. Nuclear energy comes from processes that convert measurable amounts of mass into energy. Nuclear energy is transformed into the energy of sunlight, into electrical energy in power plants, and into the energy of the heat transfer and blast in weapons. Atoms and molecules inside all objects are in random motion. This internal mechanical energy from the random motions is called thermal energy , because it is related to the temperature of the object. These and all other forms of energy can be converted into one another and can do work.
Table gives the amount of energy stored, used, or released from various objects and in various phenomena. The range of energies and the variety of types and situations is impressive.
Problem-Solving Strategies for Energy
You will find the following problem-solving strategies useful whenever you deal with energy. The strategies help in organizing and reinforcing energy concepts. In fact, they are used in the examples presented in this chapter. The familiar general problem-solving strategies presented earlier—involving identifying physical principles, knowns, and unknowns, checking units, and so on —continue to be relevant here.
Step 1. Determine the system of interest and identify what information is given and what quantity is to be calculated. A sketch will help.
Step 2. Examine all the forces involved and determine whether you know or are given the potential energy from the work done by the forces. Then use step 3 or step 4.
Step 3. If you know the potential energies for the forces that enter into the problem, then forces are all conservative, and you can apply conservation of mechanical energy simply in terms of potential and kinetic energy. The equation expressing conservation of energy is
\[KE_i + PE_i = KE_f + PE_f.\]
Step 4. If you know the potential energy for only some of the forces, possibly because some of them are nonconservative and do not have a potential energy, or if there are other energies that are not easily treated in terms of force and work, then the conservation of energy law in its most general form must be used.
\[KE_i + PE_i + W_{nc} + OE_i = KE_f + PE_f + OE_f.\]
In most problems, one or more of the terms is zero, simplifying its solution. Do not calculate \(W_c\), the work done by conservative forces; it is already incorporated in the \(PE\) terms.
Step 5. You have already identified the types of work and energy involved (in step 2). Before solving for the unknown, eliminate terms wherever possible to simplify the algebra. For example, choose \(h = 0\) at either the initial or final point, so that \(PE_g\) is zero there. Then solve for the unknown in the customary manner.
Step 6. Check the answer to see if it is reasonable . Once you have solved a problem, reexamine the forms of work and energy to see if you have set up the conservation of energy equation correctly. For example, work done against friction should be negative, potential energy at the bottom of a hill should be less than that at the top, and so on. Also check to see that the numerical value obtained is reasonable. For example, the final speed of a skateboarder who coasts down a 3-m-high ramp could reasonably be 20 km/h, but not 80 km/h.

Transformation of Energy
The transformation of energy from one form into others is happening all the time. The chemical energy in food is converted into thermal energy through metabolism; light energy is converted into chemical energy through photosynthesis. In a larger example, the chemical energy contained in coal is converted into thermal energy as it burns to turn water into steam in a boiler. This thermal energy in the steam in turn is converted to mechanical energy as it spins a turbine, which is connected to a generator to produce electrical energy. (In all of these examples, not all of the initial energy is converted into the forms mentioned. This important point is discussed later in this section.)
Another example of energy conversion occurs in a solar cell. Sunlight impinging on a solar cell (Figure 7.7.1) produces electricity, which in turn can be used to run an electric motor. Energy is converted from the primary source of solar energy into electrical energy and then into mechanical energy.

Even though energy is conserved in an energy conversion process, the output of useful energy or work will be less than the energy input. The efficiency \(E_{ff}\) of an energy conversion process is defined as
\[Efficiency \, (E_{ff}) = \dfrac{useful \, energy \, or \, work \, output}{total \, energy \, input} = \dfrac{W_{out}}{E_{in}}.\]
Table lists some efficiencies of mechanical devices and human activities. In a coal-fired power plant, for example, about 40% of the chemical energy in the coal becomes useful electrical energy. The other 60% transforms into other (perhaps less useful) energy forms, such as thermal energy, which is then released to the environment through combustion gases and cooling towers.
Efficiency of the Human Body and Mechanical Devices
PhET Explorations: Masses and Springs
A realistic mass and spring laboratory. Hang masses from springs and adjust the spring stiffness and damping. You can even slow time. Transport the lab to different planets. A chart shows the kinetic, potential, and thermal energies for each spring.
- The law of conservation of energy states that the total energy is constant in any process. Energy may change in form or be transferred from one system to another, but the total remains the same.
- When all forms of energy are considered, conservation of energy is written in equation form as \[KE_i + PE_i + W_{nc} + OE_i = KE_f + PE_f + OE_f ,\] where \(OE\) is all other forms of energy besides mechanical energy.
- Commonly encountered forms of energy include electric energy, chemical energy, radiant energy, nuclear energy, and thermal energy.
- Energy is often utilized to do work, but it is not possible to convert all the energy of a system to work.
The efficiency \(E_{ff}\) of a machine or human is defined to be \(E_{ff} = \frac{W_{out}}{E_{in}},\) where \(W_{out}\) is useful work output and \(E_{in}\) s the energy consumed.
- Law Of Conservation Of Energy
Law of Conservation of Energy
Energy is required for the evolution of life forms on earth. In physics, it is defined as the capacity to do work. We know that energy exists in different forms in nature. You have learned about various forms of energy – heat, electrical, chemical, nuclear, etc. In this article, we will learn about the laws and principles that govern energy. This law is known as the law of conservation of energy.
What is the Law of Conservation of Energy?
The law of conservation of energy states that energy can neither be created nor be destroyed. Although, it may be transformed from one form to another. If you take all forms of energy into account, the total energy of an isolated system always remains constant. All the forms of energy follow the law of conservation of energy. In brief, the law of conservation of energy states that
In a closed system, i.e., a system that is isolated from its surroundings, the total energy of the system is conserved.
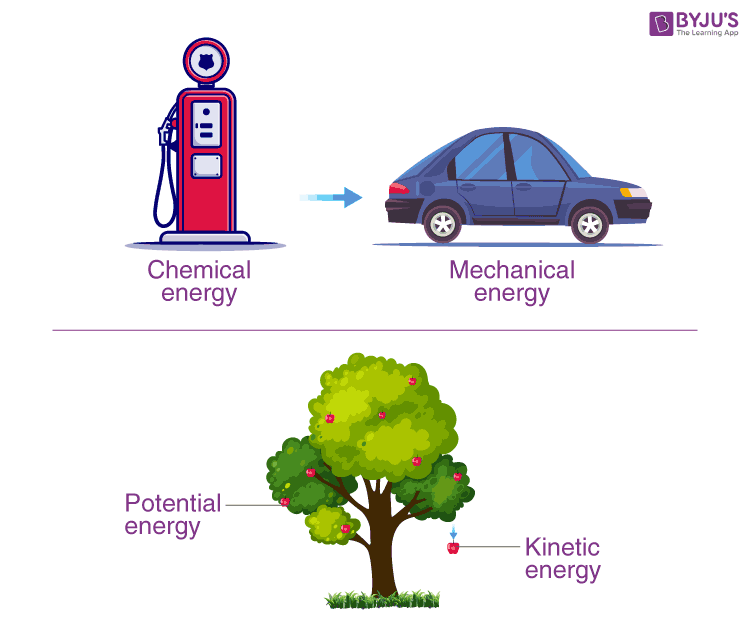
Example of Energy Transformation
So in an isolated system such as the universe, if there is a loss of energy in some part of it, there must be a gain of an equal amount of energy in some other part of the universe. Although this principle cannot be proved, there is no known example of a violation of the principle of conservation of energy.
The amount of energy in any system is determined by the following equation: U T = U i + W + Q
- U T is the total energy of a system
- U i is the initial energy of a system
- Q is the heat added or removed from the system
- W is the work done by or on the system
The change in the internal energy of the system is determined using the equation ΔU = W + Q
Suggested Reading
Law of Conservation of Energy Derivation
Considering the potential energy at the surface of the earth to be zero. Let us see an example of a fruit falling from a tree.
Consider a point A, which is at height ‘H’ from the ground on the tree, the velocity of the fruit is zero hence potential energy is maximum there.
E = mgH ———- (1)
When the fruit falls, its potential energy decreases, and kinetic energy increases.
At point B, which is near the bottom of the tree, the fruit is falling freely under gravity and is at a height X from the ground, and it has speed as it reaches point B. So, at this point, it will have both kinetic and potential energy.
E = K.E + P.E
P.E = mgX ——— (2)
According to the third equation of motion,
\(\begin{array}{l}v^{2 }= 2g(H – X)\\ \\ \Rightarrow \frac{1}{2}mv^{2}=\frac{1}{2}m.2g(H – X)\\ \\ \Rightarrow K.E=\frac{1}{2}m.2g(H – X) \\ \Rightarrow K.E=mg(H – X)\end{array} \)
K.E=mg(H-X)——– (3)
Using (1), (2) and (3)
E = mg(H – X) + mgX
E = mg(H – X + X)
Similarly, if we see the energy at point C, which is at the bottom of the tree, it will come out to be mgH. We can see as the fruit is falling to the bottom, here, potential energy is getting converted into kinetic energy. So there must be a point where kinetic energy becomes equal to potential energy. Suppose we need to find that height ‘x’ from the ground. We know at that point,
As the body is at height X from the ground,
P.E = mgX ——— (5)
Using (4) and (5) we get,
\(\begin{array}{l}mgX=\frac{mgH}{2}\\ \\ \Rightarrow X=\frac{H}{2}\end{array} \)
H/2 is referred to as the new height.
Read more: Potential Energy
You may want to watch the following video on potential energy and kinetic energy to understand better the principle of conservation of energy

Energy Conservation:
Energy conservation is not about limiting the use of resources which will finally run out altogether. The ideal way of conservation would be reducing demand on a limited supply and enabling that supply to begin to rebuild itself. Many times the best way of doing this is to replace the energy used with an alternative.
Read more: Energy Conservation
Law of Conservation of Energy Examples:
In Physics, most of the inventions rely on the fact that energy is conserved when it is transferred from one form to another. A number of electrical and mechanical devices operate solely on the law of conservation of energy. We will discuss a few examples here.
- In a torch, the chemical energy of the batteries is converted into electrical energy, which is converted into light and heat energy.
- In hydroelectric power plants, waterfalls on the turbines from a height. This, in turn, rotates the turbines and generates electricity. Hence, the potential energy of water is converted into the kinetic energy of the turbine, which is further converted into electrical energy.
- In a loudspeaker, electrical energy is converted into sound energy.
- In a microphone, sound energy is converted into electrical energy.
- In a generator, mechanical energy is converted into electrical energy.
- When fuels are burnt, chemical energy is converted into heat and light energy.
- Chemical energy from food is converted to thermal energy when it is broken down in the body and is used to keep it warm.
Read more: Energy resources
The below video provides Work and Energy quiz

Frequently Asked Questions – FAQs
What is energy.
Energy is the ability to do work.
State the law of conservation of energy?
Which types of energy can be seen when a block slides down a slope, give an example where potential energy is converted into kinetic energy, give an example to prove energy is conserved.
A moving car proves that potential energy is converted into kinetic energy.
Watch the video and solve complete exemplar questions in the chapter Work and Energy Class 9

Stay tuned with BYJU’S to learn more about the law of conservation of energy, heat energy, and much more.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Physics related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Who was the propounder of energy conservation law ?? Jaims Prescott jule or Einstein????
James Prescott Joule set the foundation for the theory of conservation of energy, which later influenced the First Law of Thermodynamics.
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
Energy Conservation of energy
The law of Conservation of Energy states that energy cannot be created or destroyed - it can only be transferred from one type to another.
Part of Physics Dynamics
Conservation of energy
This video can not be played
To play this video you need to enable JavaScript in your browser.
Conservation of energy – electrical
One of the fundamental laws of nature is that energy cannot be made or destroyed, just transformed from one form into another.
If you think about it, you require energy to run about. That energy comes from your food which by one means or another has got its energy from the sun (plants by photosynthesis close photosynthesis A chemical process used by plants to make glucose and oxygen from carbon dioxide and water, using light energy. Oxygen is produced as a by-product of photosynthesis. Algae subsumed within plants and some bacteria are also photosynthetic. , animals by eating plants).
However, when you convert from one form of energy into another not all of the energy you begin with is transformed into the useful energy. Some energy will be transformed into unwanted types of energy, ie it is wasted. These unwanted types of energy reduce the amount of useful energy which is transformed during a process.
For example, a car engine converts chemical energy into kinetic energy to allow it to move – but there are several other forms of energy involved in the process, with some energy being wasted (or ‘lost’) because it is transformed to heat and sound by the engine. The amount of useful energy (in a car, this is mainly kinetic energy) is less than the amount of energy contained in the fuel. The efficiency of the process is less than 100% because of these ‘energy losses’.
More guides on this topic
- Exam skills
- Vectors and scalars
- Velocity-time graphs
- Acceleration
- Newton's Laws
- Projectile motion
Related links
- BBC Science
- BBC News: Science
- SQA National 5 Physics
- Physics.org
- The Physics Classroom
- Physics Central

IMAGES
VIDEO
COMMENTS
View 01.04 Law of Conservation of Energy.docx from CIVICS 02 at Florida Virtual School. Law of Conservation of Energy Lab Report Instructions: In Part One of the Law of Conservation of Energy lab, ... In this assignment you will be determining an operating and cash budget summary for an event company. Please complete this assignment using the ...
The purpose of this lab is to explore the law of conservation of energy and apply the engineering design process to design a model that demonstrates the law of conservation of energy. Part One: Research the Energy Skate Park Basics: Intro Simulation. Instructions: Select the Intro Simulation located at the bottom of the simulation window.
The purpose of this lab is to explore the law of conservation of energy and apply the engineering design process to design a model that demonstrates the law of conservation of energy. Part One: Research the Energy Skate Park Basics: Intro Simulation (8 points) Instructions: 1. Select the Intro Simulation located at the bottom of the simulation ...
Antonio Garcia 12/19/2017 01.04 Law of Conservation of Energy What is the Law of conservation Energy: The law of conservation of energy, a fundamental concept of physics, states that the total amount of energy remains constant in an isolated system. It implies that energy can neither be created or destroyed, but can be change from one form to another.
Conservation of energy is one of the few universal principles of physics. No exception has ever been found. It applies to physical, chemical, and biological systems. Also from page 187: "Some problems can be solved using either energy conservation or Newton's second law. Usually the energy method is easier." Using Newton's second law
is change in kinetic energy, and Δ U. . is change in potential energy. The initial mechanical energy of a system equals the final mechanical energy for a system where no work is done by non-conservative forces (conservation of mechanical energy principle). K 0 + U 0 + W NC = K + U or W NC = Δ K + Δ U. . K 0.
The efficiency Eff Eff of an energy conversion process is defined as. Efficiency(Eff)= useful energy or work output total energy input = Wout Ein. Efficiency ( Eff) = useful energy or work output total energy input = W out E in. 7.68. Table 7.2 lists some efficiencies of mechanical devices and human activities.
Energy is conserved into a closed system. You can think of this as an imaginary closed box where nothing goes in or out. The only way it can remain the same is if it doesn't transforminto a different energy. Welcome to Module 1, lesson 1.04. In this lesson we learned about the Law of Conservation of Energy, increases and decreases in energy ...
The law of conservation of energy is a physical law that states that the total energy of an isolated system is a constant, although energy can change forms.In other words, energy is conserved over time. The law of conservation of energy is the first law of thermodynamics.French mathematician and philosopher Émilie du Châtelet first proposed and tested the law in the 18th century.
Summary. The law of conservation of energy states that the total energy is constant in any process. Energy may change in form or be transferred from one system to another, but the total remains the same. When all forms of energy are considered, conservation of energy is written in equation form as.
01.04 Law of Conservation of Energy What is the Law of conservation Energy?: The law of conservation of energy, is a fundamental concept of physics, that states that the total amount of energy remains constant in an isolated system. It implies that energy can neither be created or destroyed, but can be change from one form to another. How does this law affect electricity?
thinking about energy involves energy! conserve. ex- turning water off when brushing teeth, riding your bike. Radiant energy. energy from the sun. energy is always,,, changing form. Study with Quizlet and memorize flashcards containing terms like brain-ergy, conserve, Radiant energy and more.
Law Of Conservation Of Energy : Guided Notes. Term. 1 / 18. Resource from which energy can be obtained and used. Click the card to flip 👆. Definition. 1 / 18. Energy Source. Click the card to flip 👆.
01 Law of Conservation of Energy Guided Notes Objectives: In the lesson you will: recognize that energy cannot be created or destroyed but may be transformed from one type to another cite evidence supporting energy transformation to demonstrate that energy cannot be created or destroyed
The law of conservation of energy states that energy can neither be created nor be destroyed. Although, it may be transformed from one form to another. If you take all forms of energy into account, the total energy of an isolated system always remains constant. All the forms of energy follow the law of conservation of energy. In brief, the law ...
01.04 Law of Conservation of Energy Lab Report Assignment Instructions: In Part One of the Law of Conservation of Energy lab, you will research the law of conservation of energy in a skate park simulation. In Part Two of the Law of Conservation of Energy lab, you will investigate the law of conservation of energy while using the engineering design process to design your own skate park ramp in ...
02:25. Conservation of energy - electrical. One of the fundamental laws of nature is that energy cannot be made or destroyed, just transformed from one form into another. If you think about it ...
The unit used for measuring the amount of energy from food. The amount of energy needed to raise the temperature of 1 gram of water by 1 degree Celsius. Study with Quizlet and memorize flashcards containing terms like Law of Conservation of Energy, Mechanical Energy, Mechanical energy includes these forms of energy and more.
View 01_04_notes-1.docx from SCIENCE 11 at Jupiter High School. 01.04 Law of Conservation of Energy Guided Notes Objectives: In the lesson you will: recognize that energy cannot be created or. AI Homework Help ... Module 7 Assignment: Part 2: SES 141: Energy in Everyday Life (2020 Spring - B).pdf. Solutions Available. Arizona State University ...
The law of conservation of energy states that the total energy in a system remains constant. Energy can be transferred from one system to another or change form, but the total amount of energy remains the same. For example, when a ball is raised to a certain height, it gains potential energy.
law of conservation of energy. energy cannot be created or destroyed; it may be transformed from one form into another. power. rate of energy transfer (watts, kilowatts) laws of therodynamics. 1. many different kinds of energy are interchangeable 2. the kind of energy in a given system can change but the total amount can't.
01.04 Law of Conservation of Energy Guided Notes Objectives: In the lesson you will: recognize that energy cannot be created or destroyed but may be transformed from one type to another cite evidence supporting energy transformation to demonstrate that energy cannot be created or destroyed Big Ideas: Key Questions and Terms Notes
a device that changes electrical energy into mechanical energy; contains an electromagnet. Electrical circuit. the path through which electricity flows. Conduction. the transfer of heat between objects that are touching, or from one part of an object to another. Convection. the transfer of heat that happens when the particles of a gas or liquid ...