Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- Ethical Considerations in Research | Types & Examples

Ethical Considerations in Research | Types & Examples
Published on October 18, 2021 by Pritha Bhandari . Revised on May 9, 2024.
Ethical considerations in research are a set of principles that guide your research designs and practices. Scientists and researchers must always adhere to a certain code of conduct when collecting data from people.
The goals of human research often include understanding real-life phenomena, studying effective treatments, investigating behaviors, and improving lives in other ways. What you decide to research and how you conduct that research involve key ethical considerations.
These considerations work to
- protect the rights of research participants
- enhance research validity
- maintain scientific or academic integrity
Table of contents
Why do research ethics matter, getting ethical approval for your study, types of ethical issues, voluntary participation, informed consent, confidentiality, potential for harm, results communication, examples of ethical failures, other interesting articles, frequently asked questions about research ethics.
Research ethics matter for scientific integrity, human rights and dignity, and collaboration between science and society. These principles make sure that participation in studies is voluntary, informed, and safe for research subjects.
You’ll balance pursuing important research objectives with using ethical research methods and procedures. It’s always necessary to prevent permanent or excessive harm to participants, whether inadvertent or not.
Defying research ethics will also lower the credibility of your research because it’s hard for others to trust your data if your methods are morally questionable.
Even if a research idea is valuable to society, it doesn’t justify violating the human rights or dignity of your study participants.
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
Before you start any study involving data collection with people, you’ll submit your research proposal to an institutional review board (IRB) .
An IRB is a committee that checks whether your research aims and research design are ethically acceptable and follow your institution’s code of conduct. They check that your research materials and procedures are up to code.
If successful, you’ll receive IRB approval, and you can begin collecting data according to the approved procedures. If you want to make any changes to your procedures or materials, you’ll need to submit a modification application to the IRB for approval.
If unsuccessful, you may be asked to re-submit with modifications or your research proposal may receive a rejection. To get IRB approval, it’s important to explicitly note how you’ll tackle each of the ethical issues that may arise in your study.
There are several ethical issues you should always pay attention to in your research design, and these issues can overlap with each other.
You’ll usually outline ways you’ll deal with each issue in your research proposal if you plan to collect data from participants.
| Voluntary participation | Your participants are free to opt in or out of the study at any point in time. |
|---|---|
| Informed consent | Participants know the purpose, benefits, risks, and funding behind the study before they agree or decline to join. |
| Anonymity | You don’t know the identities of the participants. Personally identifiable data is not collected. |
| Confidentiality | You know who the participants are but you keep that information hidden from everyone else. You anonymize personally identifiable data so that it can’t be linked to other data by anyone else. |
| Potential for harm | Physical, social, psychological and all other types of harm are kept to an absolute minimum. |
| Results communication | You ensure your work is free of or research misconduct, and you accurately represent your results. |
Voluntary participation means that all research subjects are free to choose to participate without any pressure or coercion.
All participants are able to withdraw from, or leave, the study at any point without feeling an obligation to continue. Your participants don’t need to provide a reason for leaving the study.
It’s important to make it clear to participants that there are no negative consequences or repercussions to their refusal to participate. After all, they’re taking the time to help you in the research process , so you should respect their decisions without trying to change their minds.
Voluntary participation is an ethical principle protected by international law and many scientific codes of conduct.
Take special care to ensure there’s no pressure on participants when you’re working with vulnerable groups of people who may find it hard to stop the study even when they want to.
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

Informed consent refers to a situation in which all potential participants receive and understand all the information they need to decide whether they want to participate. This includes information about the study’s benefits, risks, funding, and institutional approval.
You make sure to provide all potential participants with all the relevant information about
- what the study is about
- the risks and benefits of taking part
- how long the study will take
- your supervisor’s contact information and the institution’s approval number
Usually, you’ll provide participants with a text for them to read and ask them if they have any questions. If they agree to participate, they can sign or initial the consent form. Note that this may not be sufficient for informed consent when you work with particularly vulnerable groups of people.
If you’re collecting data from people with low literacy, make sure to verbally explain the consent form to them before they agree to participate.
For participants with very limited English proficiency, you should always translate the study materials or work with an interpreter so they have all the information in their first language.
In research with children, you’ll often need informed permission for their participation from their parents or guardians. Although children cannot give informed consent, it’s best to also ask for their assent (agreement) to participate, depending on their age and maturity level.
Anonymity means that you don’t know who the participants are and you can’t link any individual participant to their data.
You can only guarantee anonymity by not collecting any personally identifying information—for example, names, phone numbers, email addresses, IP addresses, physical characteristics, photos, and videos.
In many cases, it may be impossible to truly anonymize data collection . For example, data collected in person or by phone cannot be considered fully anonymous because some personal identifiers (demographic information or phone numbers) are impossible to hide.
You’ll also need to collect some identifying information if you give your participants the option to withdraw their data at a later stage.
Data pseudonymization is an alternative method where you replace identifying information about participants with pseudonymous, or fake, identifiers. The data can still be linked to participants but it’s harder to do so because you separate personal information from the study data.
Confidentiality means that you know who the participants are, but you remove all identifying information from your report.
All participants have a right to privacy, so you should protect their personal data for as long as you store or use it. Even when you can’t collect data anonymously, you should secure confidentiality whenever you can.
Some research designs aren’t conducive to confidentiality, but it’s important to make all attempts and inform participants of the risks involved.
As a researcher, you have to consider all possible sources of harm to participants. Harm can come in many different forms.
- Psychological harm: Sensitive questions or tasks may trigger negative emotions such as shame or anxiety.
- Social harm: Participation can involve social risks, public embarrassment, or stigma.
- Physical harm: Pain or injury can result from the study procedures.
- Legal harm: Reporting sensitive data could lead to legal risks or a breach of privacy.
It’s best to consider every possible source of harm in your study as well as concrete ways to mitigate them. Involve your supervisor to discuss steps for harm reduction.
Make sure to disclose all possible risks of harm to participants before the study to get informed consent. If there is a risk of harm, prepare to provide participants with resources or counseling or medical services if needed.
Some of these questions may bring up negative emotions, so you inform participants about the sensitive nature of the survey and assure them that their responses will be confidential.
The way you communicate your research results can sometimes involve ethical issues. Good science communication is honest, reliable, and credible. It’s best to make your results as transparent as possible.
Take steps to actively avoid plagiarism and research misconduct wherever possible.
Plagiarism means submitting others’ works as your own. Although it can be unintentional, copying someone else’s work without proper credit amounts to stealing. It’s an ethical problem in research communication because you may benefit by harming other researchers.
Self-plagiarism is when you republish or re-submit parts of your own papers or reports without properly citing your original work.
This is problematic because you may benefit from presenting your ideas as new and original even though they’ve already been published elsewhere in the past. You may also be infringing on your previous publisher’s copyright, violating an ethical code, or wasting time and resources by doing so.
In extreme cases of self-plagiarism, entire datasets or papers are sometimes duplicated. These are major ethical violations because they can skew research findings if taken as original data.
You notice that two published studies have similar characteristics even though they are from different years. Their sample sizes, locations, treatments, and results are highly similar, and the studies share one author in common.
Research misconduct
Research misconduct means making up or falsifying data, manipulating data analyses, or misrepresenting results in research reports. It’s a form of academic fraud.
These actions are committed intentionally and can have serious consequences; research misconduct is not a simple mistake or a point of disagreement about data analyses.
Research misconduct is a serious ethical issue because it can undermine academic integrity and institutional credibility. It leads to a waste of funding and resources that could have been used for alternative research.
Later investigations revealed that they fabricated and manipulated their data to show a nonexistent link between vaccines and autism. Wakefield also neglected to disclose important conflicts of interest, and his medical license was taken away.
This fraudulent work sparked vaccine hesitancy among parents and caregivers. The rate of MMR vaccinations in children fell sharply, and measles outbreaks became more common due to a lack of herd immunity.
Research scandals with ethical failures are littered throughout history, but some took place not that long ago.
Some scientists in positions of power have historically mistreated or even abused research participants to investigate research problems at any cost. These participants were prisoners, under their care, or otherwise trusted them to treat them with dignity.
To demonstrate the importance of research ethics, we’ll briefly review two research studies that violated human rights in modern history.
These experiments were inhumane and resulted in trauma, permanent disabilities, or death in many cases.
After some Nazi doctors were put on trial for their crimes, the Nuremberg Code of research ethics for human experimentation was developed in 1947 to establish a new standard for human experimentation in medical research.
In reality, the actual goal was to study the effects of the disease when left untreated, and the researchers never informed participants about their diagnoses or the research aims.
Although participants experienced severe health problems, including blindness and other complications, the researchers only pretended to provide medical care.
When treatment became possible in 1943, 11 years after the study began, none of the participants were offered it, despite their health conditions and high risk of death.
Ethical failures like these resulted in severe harm to participants, wasted resources, and lower trust in science and scientists. This is why all research institutions have strict ethical guidelines for performing research.
If you want to know more about statistics , methodology , or research bias , make sure to check out some of our other articles with explanations and examples.
- Normal distribution
- Measures of central tendency
- Chi square tests
- Confidence interval
- Quartiles & Quantiles
- Cluster sampling
- Stratified sampling
- Thematic analysis
- Cohort study
- Peer review
- Ethnography
Research bias
- Implicit bias
- Cognitive bias
- Conformity bias
- Hawthorne effect
- Availability heuristic
- Attrition bias
- Social desirability bias
Ethical considerations in research are a set of principles that guide your research designs and practices. These principles include voluntary participation, informed consent, anonymity, confidentiality, potential for harm, and results communication.
Scientists and researchers must always adhere to a certain code of conduct when collecting data from others .
These considerations protect the rights of research participants, enhance research validity , and maintain scientific integrity.
Research ethics matter for scientific integrity, human rights and dignity, and collaboration between science and society. These principles make sure that participation in studies is voluntary, informed, and safe.
Anonymity means you don’t know who the participants are, while confidentiality means you know who they are but remove identifying information from your research report. Both are important ethical considerations .
You can only guarantee anonymity by not collecting any personally identifying information—for example, names, phone numbers, email addresses, IP addresses, physical characteristics, photos, or videos.
You can keep data confidential by using aggregate information in your research report, so that you only refer to groups of participants rather than individuals.
These actions are committed intentionally and can have serious consequences; research misconduct is not a simple mistake or a point of disagreement but a serious ethical failure.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Bhandari, P. (2024, May 09). Ethical Considerations in Research | Types & Examples. Scribbr. Retrieved July 2, 2024, from https://www.scribbr.com/methodology/research-ethics/
Is this article helpful?

Pritha Bhandari
Other students also liked, data collection | definition, methods & examples, what is self-plagiarism | definition & how to avoid it, how to avoid plagiarism | tips on citing sources, get unlimited documents corrected.
✔ Free APA citation check included ✔ Unlimited document corrections ✔ Specialized in correcting academic texts
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
- Research ethics
- Research integrity
- Get an email alert for Research ethics
- Get the RSS feed for Research ethics
Showing 1 - 13 of 535
View by: Cover Page List Articles
Sort by: Recent Popular
Green sustainability in the hotel sector: The role of CSR, intrinsic green motivation, and personal environmental norms
Zhihong Meng, Saad Mahmood Bhatti, [ ... ], Mohammad Adnan
Be careful what you wish for: Individuals perceived to desire status are afforded less status
Andrew L. Choi, Cameron Anderson
Effect of implementing of the IDEAL discharge model on satisfaction of patient referred to trauma emergency department
Zahra Moradi Rekabdar Kalaiee, Raziyeh Ghafouri, Mitra Zandi, Malihe Nasiri
Self-certification: A novel method for increasing sharing discernment on social media
Piers Douglas Lionel Howe, Andrew Perfors, [ ... ], Sihan Dong
Co-production of an online research and resource platform for improving the health of young people—The hype project
Cerisse Gunasinghe, Nicol Bergou, [ ... ], Stephani L. Hatch
A taxing problem: The impacts of research payment practices on participants and inclusive research
Leslie E. Wolf, Samantha Kench, Christy J. W. Ledford
Necator americanus (hookworm) in areas of active hookworm transmission in Brazil">Public engagement for the conduct of a controlled human infection study testing vaccines against Necator americanus (hookworm) in areas of active hookworm transmission in Brazil
Luciene Barra Ribeiro, Andréa Gazzinelli, [ ... ], Maria Flávia Gazzinelli Bethony
Women, peace and insecurity: The risks of peacebuilding in everyday life for women in Sri Lanka and Nepal
Karen Brounéus, Erika Forsberg, [ ... ], Maneesha Wanasinghe-Pasqual
Cross-border data sharing through the lens of research ethics committee members in sub-Saharan Africa
Nezerith Cengiz, Siti M. Kabanda, Keymanthri Moodley
The role of pain self-efficacy and pain catastrophising in the relationship between chronic pain and depression: A moderated mediation model
Lauren Kardash, Cindy Lee Wall, Mal Flack, Amelia Searle
Comparison of the finishing shot and ending zone of points in Grand Slam matches of women’s doubles tennis: A cross-sectional study
Marcos Borderias, Xavier Iglesias, Rafael Martínez-Gallego, Ernest Baiget
Observation system for the technical-tactical analysis of judo by the Rio 2016 Olympic champions
David Soriano, Rafael Tarragó, [ ... ], Xavier Iglesias

Tande nou gwonde ! (Hear us roar!)- Youth perspectives of maternal near-misses: Protocol for a photovoice study of young childbearing people’s perspectives of maternal near-misses in northwest Haiti"> Tande nou gwonde ! (Hear us roar!)- Youth perspectives of maternal near-misses: Protocol for a photovoice study of young childbearing people’s perspectives of maternal near-misses in northwest Haiti
Tonya MacDonald, Marie-Carmèle Charles, [ ... ], Lawrence Mbuagbaw
Connect with Us
- PLOS ONE on Twitter
- PLOS on Facebook
- Original article
- Open access
- Published: 13 July 2021
Assisting you to advance with ethics in research: an introduction to ethical governance and application procedures
- Shivadas Sivasubramaniam 1 ,
- Dita Henek Dlabolová 2 ,
- Veronika Kralikova 3 &
- Zeenath Reza Khan 3
International Journal for Educational Integrity volume 17 , Article number: 14 ( 2021 ) Cite this article
18k Accesses
12 Citations
4 Altmetric
Metrics details
Ethics and ethical behaviour are the fundamental pillars of a civilised society. The focus on ethical behaviour is indispensable in certain fields such as medicine, finance, or law. In fact, ethics gets precedence with anything that would include, affect, transform, or influence upon individuals, communities or any living creatures. Many institutions within Europe have set up their own committees to focus on or approve activities that have ethical impact. In contrast, lesser-developed countries (worldwide) are trying to set up these committees to govern their academia and research. As the first European consortium established to assist academic integrity, European Network for Academic Integrity (ENAI), we felt the importance of guiding those institutions and communities that are trying to conduct research with ethical principles. We have established an ethical advisory working group within ENAI with the aim to promote ethics within curriculum, research and institutional policies. We are constantly researching available data on this subject and committed to help the academia to convey and conduct ethical behaviour. Upon preliminary review and discussion, the group found a disparity in understanding, practice and teaching approaches to ethical applications of research projects among peers. Therefore, this short paper preliminarily aims to critically review the available information on ethics, the history behind establishing ethical principles and its international guidelines to govern research.
The paper is based on the workshop conducted in the 5th International conference Plagiarism across Europe and Beyond, in Mykolas Romeris University, Lithuania in 2019. During the workshop, we have detailed a) basic needs of an ethical committee within an institution; b) a typical ethical approval process (with examples from three different universities); and c) the ways to obtain informed consent with some examples. These are summarised in this paper with some example comparisons of ethical approval processes from different universities. We believe this paper will provide guidelines on preparing and training both researchers and research students in appropriately upholding ethical practices through ethical approval processes.
Introduction
Ethics and ethical behaviour (often linked to “responsible practice”) are the fundamental pillars of a civilised society. Ethical behaviour with integrity is important to maintain academic and research activities. It affects everything we do, and gets precedence with anything that would include/affect, transform, or impact upon individuals, communities or any living creatures. In other words, ethics would help us improve our living standards (LaFollette, 2007 ). The focus on ethical behaviour is indispensable in certain fields such as medicine, finance, or law, but is also gaining recognition in all disciplines engaged in research. Therefore, institutions are expected to develop ethical guidelines in research to maintain quality, initiate/own integrity and above all be transparent to be successful by limiting any allegation of misconduct (Flite and Harman, 2013 ). This is especially true for higher education organisations that promote research and scholarly activities. Many European institutions have developed their own regulations for ethics by incorporating international codes (Getz, 1990 ). The lesser developed countries are trying to set up these committees to govern their academia and research. World Health Organization has stated that adhering to “ ethical principles … [is central and important]... in order to protect the dignity, rights and welfare of research participants ” (WHO, 2021 ). Ethical guidelines taught to students can help develop ethical researchers and members of society who uphold values of ethical principles in practice.
As the first European-wide consortium established to assist academic integrity (European Network for Academic Integrity – ENAI), we felt the importance of guiding those institutions and communities that are trying to teach, research, and include ethical principles by providing overarching understanding of ethical guidelines that may influence policy. Therefore, we set up an advisory working group within ENAI in 2018 to support matters related to ethics, ethical committees and assisting on ethics related teaching activities.
Upon preliminary review and discussion, the group found a disparity in understanding, practice and teaching approaches to ethical applications among peers. This became the premise for this research paper. We first carried out a literature survey to review and summarise existing ethical governance (with historical perspectives) and procedures that are already in place to guide researchers in different discipline areas. By doing so, we attempted to consolidate, document and provide important steps in a typical ethical application process with example procedures from different universities. Finally, we attempted to provide insights and findings from practical workshops carried out at the 5th International Conference Plagiarism across Europe and Beyond, in Mykolas Romeris University, Lithuania in 2019, focussing on:
• highlighting the basic needs of an ethical committee within an institution,
• discussing and sharing examples of a typical ethical approval process,
• providing guidelines on the ways to teach research ethics with some examples.
We believe this paper provides guidelines on preparing and training both researchers and research students in appropriately upholding ethical practices through ethical approval processes.
Background literature survey
Responsible research practice (RRP) is scrutinised by the aspects of ethical principles and professional standards (WHO’s Code of Conduct for responsible Research, 2017). The Singapore statement on research integrity (The Singapore Statement on Research integrity, 2010) has provided an internationally acceptable guidance for RRP. The statement is based on maintaining honesty, accountability, professional courtesy in all aspects of research and maintaining fairness during collaborations. In other words, it does not simply focus on the procedural part of the research, instead covers wider aspects of “integrity” beyond the operational aspects (Israel and Drenth, 2016 ).
Institutions should focus on providing ethical guidance based on principles and values reflecting upon all aspects/stages of research (from the funding application/project development stage upto or beyond project closing stage). Figure 1 summarizes the different aspects/stages of a typical research and highlights the needs of RRP in compliance with ethical governance at each stage with examples (the figure is based on Resnik, 2020 ; Žukauskas et al., 2018 ; Anderson, 2011 ; Fouka and Mantzorou, 2011 ).
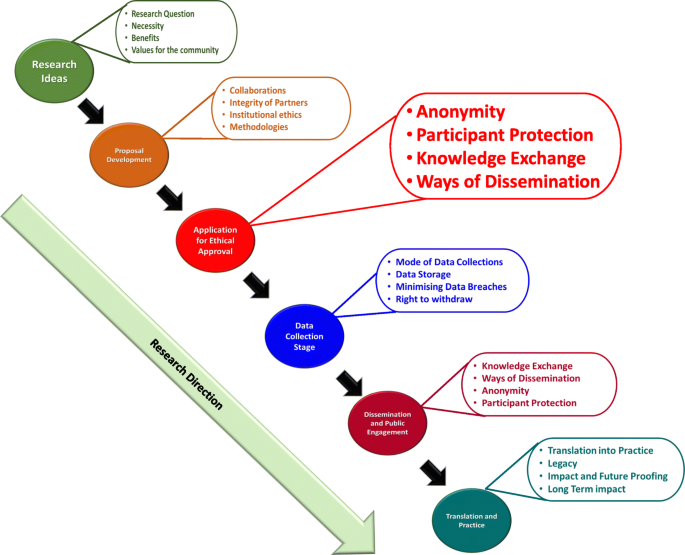
Summary of the enabling ethical governance at different stages of research. Note that it is imperative for researchers to proactively consider the ethical implications before, during and after the actual research process. The summary shows that RRP should be in line with ethical considerations even long before the ethical approval stage
Individual responsibilities to enhance RRP
As explained in Fig. 1 , a successfully governed research should consider ethics at the planning stages prior to research. Many international guidance are compatible in enforcing/recommending 14 different “responsibilities” that were first highlighted in the Singapore Statement (2010) for researchers to follow and achieve competency in RRP. In order to understand the purpose and the expectation of these ethical guidelines, we have carried out an initial literature survey on expected individual responsibilities. These are summarised in Table 1 .
By following these directives, researchers can carry out accountable research by maximising ethical self-governance whilst minimising misconducts. In our own experiences of working with many researchers, their focus usually revolves around ethical “clearance” rather than behaviour. In other words, they perceive this as a paper exercise rather than trying to “own” ethical behaviour in everything they do. Although the ethical principles and responsibilities are explicitly highlighted in the majority of international guidelines [such as UK’s Research Governance Policy (NICE, 2018 ), Australian Government’s National Statement on Ethical Conduct in Human Research (Difn website a - National Statement on Ethical Conduct in Human Research (NSECHR), 2018 ), the Singapore Statement (2010) etc.]; and the importance of holistic approach has been argued in ethical decision making, many researchers and/or institutions only focus on ethics linked to the procedural aspects.
Studies in the past have also highlighted inconsistencies in institutional guidelines pointing to the fact that these inconsistencies may hinder the predicted research progress (Desmond & Dierickx 2021 ; Alba et al., 2020 ; Dellaportas et al., 2014 ; Speight 2016 ). It may also be possible that these were and still are linked to the institutional perceptions/expectations or the pre-empting contextual conditions that are imposed by individual countries. In fact, it is interesting to note many research organisations and HE institutions establish their own policies based on these directives.
Research governance - origins, expectations and practices
Ethical governance in clinical medicine helps us by providing a structure for analysis and decision-making. By providing workable definitions of benefits and risks as well as the guidance for evaluating/balancing benefits over risks, it supports the researchers to protect the participants and the general population.
According to the definition given by National Institute of Clinical care Excellence, UK (NICE 2018 ), “ research governance can be defined as the broad range of regulations, principles and standards of good practice that ensure high quality research ”. As stated above, our literature-based research survey showed that most of the ethical definitions are basically evolved from the medical field and other disciplines have utilised these principles to develop their own ethical guidance. Interestingly, historical data show that the medical research has been “self-governed” or in other words implicated by the moral behaviour of individual researchers (Fox 2017 ; Shaw et al., 2005 ; Getz, 1990 ). For example, early human vaccination trials conducted in 1700s used the immediate family members as test subjects (Fox, 2017 ). Here the moral justification might have been the fact that the subjects who would have been at risk were either the scientists themselves or their immediate families but those who would reap the benefits from the vaccination were the general public/wider communities. However, according to the current ethical principles, this assumption is entirely not acceptable.
Historically, ambiguous decision-making and resultant incidences of research misconduct have led to the need for ethical research governance in as early as the 1940’s. For instance, the importance of an international governance was realised only after the World War II, when people were astonished to note the unethical research practices carried out by Nazi scientists. As a result of this, in 1947 the Nuremberg code was published. The code mainly focussed on the following:
Informed consent and further insisted the research involving humans should be based on prior animal work,
The anticipated benefits should outweigh the risk,
Research should be carried out only by qualified scientists must conduct research,
Avoiding physical and mental suffering and.
Avoiding human research that would result in which death or disability.
(Weindling, 2001 ).
Unfortunately, it was reported that many researchers in the USA and elsewhere considered the Nuremberg code as a document condemning the Nazi atrocities, rather than a code for ethical governance and therefore ignored these directives (Ghooi, 2011 ). It was only in 1964 that the World Medical Association published the Helsinki Declaration, which set the stage for ethical governance and the implementation of the Institutional Review Board (IRB) process (Shamoo and Irving, 1993 ). This declaration was based on Nuremberg code. In addition, the declaration also paved the way for enforcing research being conducted in accordance with these guidelines.
Incidentally, the focus on research/ethical governance gained its momentum in 1974. As a result of this, a report on ethical principles and guidelines for the protection of human subjects of research was published in 1979 (The Belmont Report, 1979 ). This report paved the way to the current forms of ethical governance in biomedical and behavioural research by providing guidance.
Since 1994, the WHO itself has been providing several guidance to health care policy-makers, researchers and other stakeholders detailing the key concepts in medical ethics. These are specific to applying ethical principles in global public health.
Likewise, World Organization for Animal Health (WOAH), and International Convention for the Protection of Animals (ICPA) provide guidance on animal welfare in research. Due to this continuous guidance, together with accepted practices, there are internationally established ethical guidelines to carry out medical research. Our literature survey further identified freely available guidance from independent organisations such as COPE (Committee of Publication Ethics) and ALLEA (All European Academics) which provide support for maintaining research ethics in other fields such as education, sociology, psychology etc. In reality, ethical governance is practiced differently in different countries. In the UK, there is a clinical excellence research governance, which oversees all NHS related medical research (Mulholland and Bell, 2005 ). Although, the governance in other disciplines is not entirely centralised, many research funding councils and organisations [such as UKRI (UK-Research and Innovation; BBSC (Biotechnology and Biological Sciences Research Council; MRC (Medical Research Council); EPSRC (Economic and Social Research Council)] provide ethical governance and expect institutional adherence and monitoring. They expect local institutional (i.e. university/institutional) research governance for day-to-day monitoring of the research conducted within the organisation and report back to these funding bodies, monthly or annually (Department of Health, 2005). Likewise, there are nationally coordinated/regulated ethics governing bodies such as the US Office for Human Research Protections (US-OHRP), National Institute of Health (NIH) and the Canadian Institutes for Health Research (CIHR) in the USA and Canada respectively (Mulholland and Bell, 2005 ). The OHRP in the USA formally reviews all research activities involving human subjects. On the other hand, in Canada, CIHR works with the Natural Sciences and Engineering Research Council (NSERC), and the Social Sciences and Humanities Research Council (SSHRC). They together have produced a Tri-Council Policy Statement (TCPS) (Stephenson et al., 2020 ) as ethical governance. All Canadian institutions are expected to adhere to this policy for conducting research. As for Australia, the research is governed by the Australian code for the responsible conduct of research (2008). It identifies the responsibilities of institutions and researchers in all areas of research. The code has been jointly developed by the National Health and Medical Research Council (NHMRC), the Australian Research Council (ARC) and Universities Australia (UA). This information is summarized in Table 2 .
Basic structure of an institutional ethical advisory committee (EAC)
The WHO published an article defining the basic concepts of an ethical advisory committee in 2009 (WHO, 2009 - see above). According to this, many countries have established research governance and monitor the ethical practice in research via national and/or regional review committees. The main aims of research ethics committees include reviewing the study proposals, trying to understand the justifications for human/animal use, weighing the merits and demerits of the usage (linking to risks vs. potential benefits) and ensuring the local, ethical guidelines are followed Difn website b - Enago academy Importance of Ethics Committees in Scholarly Research, 2020 ; Guide for Research Ethics - Council of Europe, 2014 ). Once the research has started, the committee needs to carry out periodic surveillance to ensure the institutional ethical norms are followed during and beyond the study. They may also be involved in setting up and/or reviewing the institutional policies.
For these aspects, IRB (or institutional ethical advisory committee - IEAC) is essential for local governance to enhance best practices. The advantage of an IRB/EEAC is that they understand the institutional conditions and can closely monitor the ongoing research, including any changes in research directions. On the other hand, the IRB may be overly supportive to accept applications, influenced by the local agenda for achieving research excellence, disregarding ethical issues (Kotecha et al., 2011 ; Kayser-Jones, 2003 ) or, they may be influenced by the financial interests in attracting external funding. In this respect, regional and national ethics committees are advantageous to ensure ethical practice. Due to their impartiality, they would provide greater consistency and legitimacy to the research (WHO, 2009 ). However, the ethical approval process of regional and national ethics committees would be time consuming, as they do not have the local knowledge.
As for membership in the IRBs, most of the guidelines [WHO, NICE, Council of Europe, (2012), European Commission - Facilitating Research Excellence in FP7 ( 2013 ) and OHRP] insist on having a variety of representations including experts in different fields of research, and non-experts with the understanding of local, national/international conflicts of interest. The former would be able to understand/clarify the procedural elements of the research in different fields; whilst the latter would help to make neutral and impartial decisions. These non-experts are usually not affiliated to the institution and consist of individuals representing the broader community (particularly those related to social, legal or cultural considerations). IRBs consisting of these varieties of representation would not only be in a position to understand the study procedures and their potential direct or indirect consequences for participants, but also be able to identify any community, cultural or religious implications of the study.
Understanding the subtle differences between ethics and morals
Interestingly, many ethical guidelines are based on society’s moral “beliefs” in such a way that the words “ethics”‘and “morals” are reciprocally used to define each other. However, there are several subtle differences between them and we have attempted to compare and contrast them herein. In the past, many authors have interchangeably used the words “morals”‘and “ethics”‘(Warwick, 2003 ; Kant, 2018 ; Hazard, GC (Jr)., 1994 , Larry, 1982 ). However, ethics is linked to rules governed by an external source such as codes of conduct in workplaces (Kuyare et al., 2014 ). In contrast, morals refer to an individual’s own principles regarding right and wrong. Quinn ( 2011 ) defines morality as “ rules of conduct describing what people ought and ought not to do in various situations … ” while ethics is “... the philosophical study of morality, a rational examination into people’s moral beliefs and behaviours ”. For instance, in a case of parents demanding that schools overturn a ban on use of corporal punishment of children by schools and teachers (Children’s Rights Alliance for England, 2005 ), the parents believed that teachers should assume the role of parent in schools and use corporal or physical punishment for children who misbehaved. This stemmed from their beliefs and what they felt were motivated by “beliefs of individuals or groups”. For example, recent media highlights about some parents opposing LGBT (Lesbian, Gay, Bisexual, and Transgender) education to their children (BBC News, 2019 ). One parent argued, “Teaching young children about LGBT at a very early stage is ‘morally’ wrong”. She argued “let them learn by themselves as they grow”. This behaviour is linked to and governed by the morals of an ethnic community. Thus, morals are linked to the “beliefs of individuals or group”. However, when it comes to the LGBT rights these are based on ethical principles of that society and governed by law of the land. However, the rights of children to be protected from “inhuman and degrading” treatment is based on the ethical principles of the society and governed by law of the land. Individuals, especially those who are working in medical or judicial professions have to follow an ethical code laid down by their profession, regardless of their own feelings, time or preferences. For instance, a lawyer is expected to follow the professional ethics and represent a defendant, despite the fact that his morals indicate the defendant is guilty.
In fact, we as a group could not find many scholarly articles clearly comparing or contrasting ethics with morals. However, a table presented by Surbhi ( 2015 ) (Difn website c ) tries to differentiate these two terms (see Table 3 ).
Although Table 3 gives some insight on the differences between these two terms, in practice many use these terms as loosely as possible mainly because of their ambiguity. As a group focussed on the application of these principles, we would recommend to use the term “ethics” and avoid “morals” in research and academia.
Based on the literature survey carried out, we were able to identify the following gaps:
there is some disparity in existing literature on the importance of ethical guidelines in research
there is a lack of consensus on what code of conduct should be followed, where it should be derived from and how it should be implemented
The mission of ENAI’s ethical advisory working group
The Ethical Advisory Working Group of ENAI was established in 2018 to promote ethical code of conduct/practice amongst higher educational organisations within Europe and beyond (European Network for Academic Integrity, 2018 ). We aim to provide unbiased advice and consultancy on embedding ethical principles within all types of academic, research and public engagement activities. Our main objective is to promote ethical principles and share good practice in this field. This advisory group aims to standardise ethical norms and to offer strategic support to activities including (but not exclusive to):
● rendering advice and assistance to develop institutional ethical committees and their regulations in member institutions,
● sharing good practice in research and academic ethics,
● acting as a critical guide to institutional review processes, assisting them to maintain/achieve ethical standards,
● collaborating with similar bodies in establishing collegiate partnerships to enhance awareness and practice in this field,
● providing support within and outside ENAI to develop materials to enhance teaching activities in this field,
● organising training for students and early-career researchers about ethical behaviours in form of lectures, seminars, debates and webinars,
● enhancing research and dissemination of the findings in matters and topics related to ethics.
The following sections focus on our suggestions based on collective experiences, review of literature provided in earlier sections and workshop feedback collected:
a) basic needs of an ethical committee within an institution;
b) a typical ethical approval process (with examples from three different universities); and
c) the ways to obtain informed consent with some examples. This would give advice on preparing and training both researchers and research students in appropriately upholding ethical practices through ethical approval processes.
Setting up an institutional ethical committee (ECs)
Institutional Ethical Committees (ECs) are essential to govern every aspect of the activities undertaken by that institute. With regards to higher educational organisations, this is vital to establish ethical behaviour for students and staff to impart research, education and scholarly activities (or everything) they do. These committees should be knowledgeable about international laws relating to different fields of studies (such as science, medicine, business, finance, law, and social sciences). The advantages and disadvantages of institutional, subject specific or common (statutory) ECs are summarised in Fig. 2 . Some institutions have developed individual ECs linked to specific fields (or subject areas) whilst others have one institutional committee that overlooks the entire ethical behaviour and approval process. There is no clear preference between the two as both have their own advantages and disadvantages (see Fig. 2 ). Subject specific ECs are attractive to medical, law and business provisions, as it is perceived the members within respective committees would be able to understand the subject and therefore comprehend the need of the proposed research/activity (Kadam, 2012 ; Schnyder et al., 2018 ). However, others argue, due to this “ specificity ”, the committee would fail to forecast the wider implications of that application. On the other hand, university-wide ECs would look into the wider implications. Yet they find it difficult to understand the purpose and the specific applications of that research. Not everyone understands dynamics of all types of research methodologies, data collection, etc., and therefore there might be a chance of a proposal being rejected merely because the EC could not understand the research applications (Getz, 1990 ).
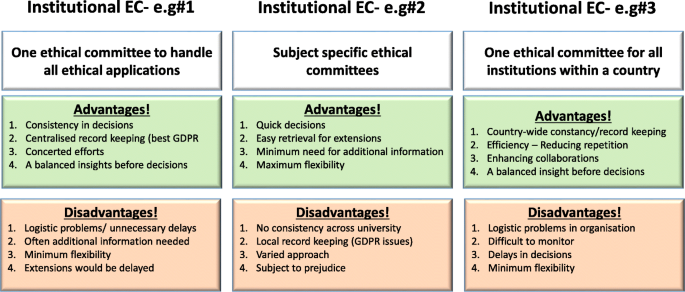
Summary of advantages and disadvantages of three different forms of ethical committees
[N/B for Fig. 2 : Examples of different types of ethical application procedures and forms used were discussed with the workshop attendees to enhance their understanding of the differences. GDPR = General Data Protection Regulation].
Although we recommend a designated EC with relevant professional, academic and ethical expertise to deal with particular types of applications, the membership (of any EC) should include some non-experts who would represent the wider community (see above). Having some non-experts in EC would not only help the researchers to consider explaining their research in layperson’s terms (by thinking outside the box) but also would ensure efficiency without compromising participants/animal safety. They may even help to address the common ethical issues outside research culture. Some UK universities usually offer this membership to a clergy, councillor or a parliamentarian who does not have any links to the institutions. Most importantly, it is vital for any EC members to undertake further training in addition to previous experience in the relevant field of research ethics.
Another issue that raises concerns is multi-centre research, involving several institutions, where institutionalised ethical approvals are needed from each partner. In some cases, such as clinical research within the UK, a common statutory EC called National Health Services (NHS) Research Ethics Committee (NREC) is in place to cover research ethics involving all partner institutions (NHS, 2018 ). The process of obtaining approval from this type of EC takes time, therefore advanced planning is needed.
Ethics approval forms and process
During the workshop, we discussed some anonymised application forms obtained from open-access sources for qualitative and quantitative research as examples. Considering research ethics, for the purpose of understanding, we arbitrarily divided this in two categories; research based on (a) quantitative and (b) qualitative methodologies. As their name suggests their research approach is extremely different from each other. The discussion elicited how ECs devise different types of ethical application form/questions. As for qualitative research, these are often conducted as “face-to-face” interviews, which would have implications on volunteer anonymity.
Furthermore, discussions posited when the interviews are replaced by on-line surveys, they have to be administered through registered university staff to maintain confidentiality. This becomes difficult when the research is a multi-centre study. These types of issues are also common in medical research regarding participants’ anonymity, confidentially, and above all their right to withdraw consent to be involved in research.
Storing and protecting data collected in the process of the study is also a point of consideration when applying for approval.
Finally, the ethical processes of invasive (involving human/animals) and non-invasive research (questionnaire based) may slightly differ from one another. Following research areas are considered as investigations that need ethical approval:
research that involves human participants (see below)
use of the ‘products’ of human participants (see below)
work that potentially impacts on humans (see below)
research that involves animals
In addition, it is important to provide a disclaimer even if an ethical approval is deemed unnecessary. Following word cloud (Fig. 3 ) shows the important variables that need to be considered at the brainstorming stage before an ethical application. It is worth noting the importance of proactive planning predicting the “unexpected” during different phases of a research project (such as planning, execution, publication, and future directions). Some applications (such as working with vulnerable individuals or children) will require safety protection clearance (such as DBS - Disclosure and Barring Service, commonly obtained from the local police). Please see section on Research involving Humans - Informed consents for further discussions.
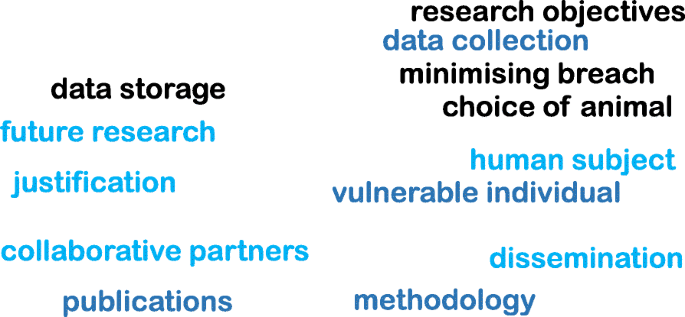
Examples of important variables that need to be considered for an ethical approval
It is also imperative to report or re-apply for ethical approval for any minor or major post-approval changes to original proposals made. In case of methodological changes, evidence of risk assessments for changes and/or COSHH (Control of Substances Hazardous to Health Regulations) should also be given. Likewise, any new collaborative partners or removal of researchers should also be notified to the IEAC.
Other findings include:
in case of complete changes in the project, the research must be stopped and new approval should be seeked,
in case of noticing any adverse effects to project participants (human or non-human), these should also be notified to the committee for appropriate clearance to continue the work, and
the completion of the project must also be notified with the indication whether the researchers may restart the project at a later stage.
Research involving humans - informed consents
While discussing research involving humans and based on literature review, findings highlight the human subjects/volunteers must willingly participate in research after being adequately informed about the project. Therefore, research involving humans and animals takes precedence in obtaining ethical clearance and its strict adherence, one of which is providing a participant information sheet/leaflet. This sheet should contain a full explanation about the research that is being carried out and be given out in lay-person’s terms in writing (Manti and Licari 2018 ; Hardicre 2014 ). Measures should also be in place to explain and clarify any doubts from the participants. In addition, there should be a clear statement on how the participants’ anonymity is protected. We provide below some example questions below to help the researchers to write this participant information sheet:
What is the purpose of the study?
Why have they been chosen?
What will happen if they take part?
What do they have to do?
What happens when the research stops?
What if something goes wrong?
What will happen to the results of the research study?
Will taking part be kept confidential?
How to handle “vulnerable” participants?
How to mitigate risks to participants?
Many institutional ethics committees expect the researchers to produce a FAQ (frequently asked questions) in addition to the information about research. Most importantly, the researchers also need to provide an informed consent form, which should be signed by each human participant. The five elements identified that are needed to be considered for an informed consent statement are summarized in Fig. 4 below (slightly modified from the Federal Policy for the Protection of Human Subjects ( 2018 ) - Diffn website c ).
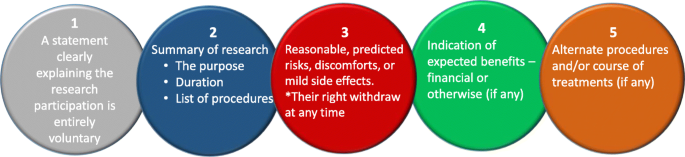
Five basic elements to consider for an informed consent [figure adapted from Diffn website c ]
The informed consent form should always contain a clause for the participant to withdraw their consent at any time. Should this happen all the data from that participant should be eliminated from the study without affecting their anonymity.
Typical research ethics approval process
In this section, we provide an example flow chart explaining how researchers may choose the appropriate application and process, as highlighted in Fig. 5 . However, it is imperative to note here that these are examples only and some institutions may have one unified application with separate sections to demarcate qualitative and quantitative research criteria.
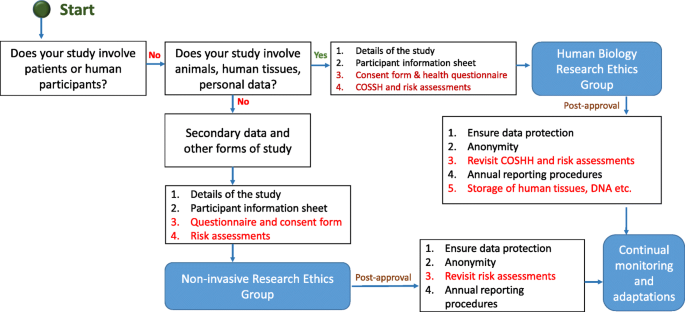
Typical ethical approval processes for quantitative and qualitative research. [N/B for Fig. 5 - This simplified flow chart shows that fundamental process for invasive and non-invasive EC application is same, the routes and the requirements for additional information are slightly different]
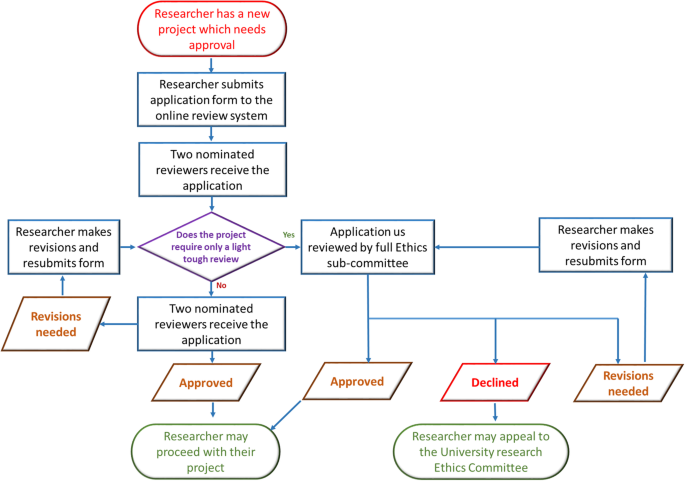
Once the ethical application is submitted, the EC should ensure a clear approval procedure with distinctly defined timeline. An example flow chart showing the procedure for an ethical approval was obtained from University of Leicester as open-access. This is presented in Fig. 6 . Further examples of the ethical approval process and governance were discussed in the workshop.
An example ethical approval procedures conducted within University of Leicester (Figure obtained from the University of Leicester research pages - Difn website d - open access)
Strategies for ethics educations for students
Student education on the importance of ethics and ethical behaviour in research and scholarly activities is extremely essential. Literature posits in the area of medical research that many universities are incorporating ethics in post-graduate degrees but when it comes to undergraduate degrees, there is less appetite to deliver modules or even lectures focussing on research ethics (Seymour et al., 2004 ; Willison and O’Regan, 2007 ). This may be due to the fact that undergraduate degree structure does not really focus on research (DePasse et al., 2016 ). However, as Orr ( 2018 ) suggested, institutions should focus more on educating all students about ethics/ethical behaviour and their importance in research, than enforcing punitive measures for unethical behaviour. Therefore, as an advisory committee, and based on our preliminary literature survey and workshop results, we strongly recommend incorporating ethical education within undergraduate curriculum. Looking at those institutions which focus on ethical education for both under-and postgraduate courses, their approaches are either (a) a lecture-based delivery, (b) case study based approach or (c) a combined delivery starting with a lecture on basic principles of ethics followed by generating a debate based discussion using interesting case studies. The combined method seems much more effective than the other two as per our findings as explained next.
As many academics who have been involved in teaching ethics and/or research ethics agree, the underlying principles of ethics is often perceived as a boring subject. Therefore, lecture-based delivery may not be suitable. On the other hand, a debate based approach, though attractive and instantly generates student interest, cannot be effective without students understanding the underlying basic principles. In addition, when selecting case studies, it would be advisable to choose cases addressing all different types of ethical dilemmas. As an advisory group within ENAI, we are in the process of collating supporting materials to help to develop institutional policies, creating advisory documents to help in obtaining ethical approvals, and teaching materials to enhance debate-based lesson plans that can be used by the member and other institutions.
Concluding remarks
In summary, our literature survey and workshop findings highlight that researchers should accept that ethics underpins everything we do, especially in research. Although ethical approval is tedious, it is an imperative process in which proactive thinking is essential to identify ethical issues that might affect the project. Our findings further lead us to state that the ethical approval process differs from institution to institution and we strongly recommend the researchers to follow the institutional guidelines and their underlying ethical principles. The ENAI workshop in Vilnius highlighted the importance of ethical governance by establishing ECs, discussed different types of ECs and procedures with some examples and highlighted the importance of student education to impart ethical culture within research communities, an area that needs further study as future scope.
Declarations
The manuscript was entirely written by the corresponding author with contributions from co-authors who have also taken part in the delivery of the workshop. Authors confirm that the data supporting the findings of this study are available within the article. We can also confirm that there are no potential competing interests with other organisations.
Availability of data and materials
Authors confirm that the data supporting the findings of this study are available within the article.
Abbreviations
ALL European academics
Australian research council
Biotechnology and biological sciences research council
Canadian institutes for health research
Committee of publication ethics
Ethical committee
European network of academic integrity
Economic and social research council
International convention for the protection of animals
institutional ethical advisory committee
Institutional review board
Immaculata university of Pennsylvania
Lesbian, gay, bisexual, and transgender
Medical research council)
National health services
National health services nih national institute of health (NIH)
National institute of clinical care excellence
National health and medical research council
Natural sciences and engineering research council
National research ethics committee
National statement on ethical conduct in human research
Responsible research practice
Social sciences and humanities research council
Tri-council policy statement
World Organization for animal health
Universities Australia
UK-research and innovation
US office for human research protections
Alba S, Lenglet A, Verdonck K, Roth J, Patil R, Mendoza W, Juvekar S, Rumisha SF (2020) Bridging research integrity and global health epidemiology (BRIDGE) guidelines: explanation and elaboration. BMJ Glob Health 5(10):e003237. https://doi.org/10.1136/bmjgh-2020-003237
Article Google Scholar
Anderson MS (2011) Research misconduct and misbehaviour. In: Bertram Gallant T (ed) Creating the ethical academy: a systems approach to understanding misconduct and empowering change in higher education. Routledge, pp 83–96
BBC News. (2019). Birmingham school LGBT LESSONS PROTEST investigated. March 8, 2019. Retrieved February 14, 2021, available online. URL: https://www.bbc.com/news/uk-england-birmingham-47498446
Children’s Rights Alliance for England. (2005). R (Williamson and others) v Secretary of State for Education and Employment. Session 2004–05. [2005] UKHL 15. Available Online. URL: http://www.crae.org.uk/media/33624/R-Williamson-and-others-v-Secretary-of-State-for-Education-and-Employment.pdf
Council of Europe. (2014). Texts of the Council of Europe on bioethical matters. Available Online. https://www.coe.int/t/dg3/healthbioethic/Texts_and_documents/INF_2014_5_vol_II_textes_%20CoE_%20bio%C3%A9thique_E%20(2).pdf
Dellaportas S, Kanapathippillai S, Khan, A and Leung, P. (2014). Ethics education in the Australian accounting curriculum: a longitudinal study examining barriers and enablers. 362–382. Available Online. URL: https://doi.org/10.1080/09639284.2014.930694 , 23, 4, 362, 382
DePasse JM, Palumbo MA, Eberson CP, Daniels AH (2016) Academic characteristics of orthopaedic surgery residency applicants from 2007 to 2014. JBJS 98(9):788–795. https://doi.org/10.2106/JBJS.15.00222
Desmond H, Dierickx K (2021) Research integrity codes of conduct in Europe: understanding the divergences. https://doi.org/10.1111/bioe.12851
Difn website a - National Statement on Ethical Conduct in Human Research (NSECHR). (2018). Available Online. URL: https://www.nhmrc.gov.au/about-us/publications/australian-code-responsible-conduct-research-2018
Difn website b - Enago academy Importance of Ethics Committees in Scholarly Research (2020, October 26). Available online. URL: https://www.enago.com/academy/importance-of-ethics-committees-in-scholarly-research/
Difn website c - Ethics vs Morals - Difference and Comparison. Retrieved July 14, 2020. Available online. URL: https://www.diffen.com/difference/Ethics_vs_Morals
Difn website d - University of Leicester. (2015). Staff ethics approval flowchart. May 1, 2015. Retrieved July 14, 2020. Available Online. URL: https://www2.le.ac.uk/institution/ethics/images/ethics-approval-flowchart/view
European Commission - Facilitating Research Excellence in FP7 (2013) https://ec.europa.eu/research/participants/data/ref/fp7/89888/ethics-for-researchers_en.pdf
European Network for Academic Integrity. (2018). Ethical advisory group. Retrieved February 14, 2021. Available online. URL: http://www.academicintegrity.eu/wp/wg-ethical/
Federal Policy for the Protection of Human Subjects. (2018). Retrieved February 14, 2021. Available Online. URL: https://www.federalregister.gov/documents/2017/01/19/2017-01058/federal-policy-for-the-protection-of-human-subjects#p-855
Flite, CA and Harman, LB. (2013). Code of ethics: principles for ethical leadership Perspect Health Inf Mana; 10(winter): 1d. PMID: 23346028
Fouka G, Mantzorou M (2011) What are the major ethical issues in conducting research? Is there a conflict between the research ethics and the nature of nursing. Health Sci J 5(1) Available Online. URL: https://www.hsj.gr/medicine/what-are-the-major-ethical-issues-in-conducting-research-is-there-a-conflict-between-the-research-ethics-and-the-nature-of-nursing.php?aid=3485
Fox G (2017) History and ethical principles. The University of Miami and the Collaborative Institutional Training Initiative (CITI) Program URL https://silo.tips/download/chapter-1-history-and-ethical-principles # (Available Online)
Getz KA (1990) International codes of conduct: An analysis of ethical reasoning. J Bus Ethics 9(7):567–577
Ghooi RB (2011) The nuremberg code–a critique. Perspect Clin Res 2(2):72–76. https://doi.org/10.4103/2229-3485.80371
Hardicre, J. (2014) Valid informed consent in research: an introduction Br J Nurs 23(11). https://doi.org/10.12968/bjon.2014.23.11.564 , 567
Hazard, GC (Jr). (1994). Law, morals, and ethics. Yale law school legal scholarship repository. Faculty Scholarship Series. Yale University. Available Online. URL: https://digitalcommons.law.yale.edu/cgi/viewcontent.cgi?referer=https://www.google.com/&httpsredir=1&article=3322&context=fss_papers
Israel, M., & Drenth, P. (2016). Research integrity: perspectives from Australia and Netherlands. In T. Bretag (Ed.), Handbook of academic integrity (pp. 789–808). Springer, Singapore. https://doi.org/10.1007/978-981-287-098-8_64
Kadam R (2012) Proactive role for ethics committees. Indian J Med Ethics 9(3):216. https://doi.org/10.20529/IJME.2012.072
Kant I (2018) The metaphysics of morals. Cambridge University Press, UK https://doi.org/10.1017/9781316091388
Kayser-Jones J (2003) Continuing to conduct research in nursing homes despite controversial findings: reflections by a research scientist. Qual Health Res 13(1):114–128. https://doi.org/10.1177/1049732302239414
Kotecha JA, Manca D, Lambert-Lanning A, Keshavjee K, Drummond N, Godwin M, Greiver M, Putnam W, Lussier M-T, Birtwhistle R (2011) Ethics and privacy issues of a practice-based surveillance system: need for a national-level institutional research ethics board and consent standards. Can Fam physician 57(10):1165–1173. https://europepmc.org/article/pmc/pmc3192088
Kuyare, MS., Taur, SR., Thatte, U. (2014). Establishing institutional ethics committees: challenges and solutions–a review of the literature. Indian J Med Ethics. https://doi.org/10.20529/IJME.2014.047
LaFollette, H. (2007). Ethics in practice (3rd edition). Blackwell
Larry RC (1982) The teaching of ethics and moral values in teaching. J High Educ 53(3):296–306. https://doi.org/10.1080/00221546.1982.11780455
Manti S, Licari A (2018) How to obtain informed consent for research. Breathe (Sheff) 14(2):145–152. https://doi.org/10.1183/20734735.001918
Mulholland MW, Bell J (2005) Research Governance and Research Funding in the USA: What the academic surgeon needs to know. J R Soc Med 98(11):496–502. https://doi.org/10.1258/jrsm.98.11.496
National Institute of Health (NIH) Ethics in Clinical Research. n.d. Available Online. URL: https://clinicalcenter.nih.gov/recruit/ethics.html
NHS (2018) Flagged Research Ethics Committees. Retrieved February 14, 2021. Available online. URL: https://www.hra.nhs.uk/about-us/committees-and-services/res-and-recs/flagged-research-ethics-committees/
NICE (2018) Research governance policy. Retrieved February 14, 2021. Available online. URL: https://www.nice.org.uk/Media/Default/About/what-we-do/science-policy-and-research/research-governance-policy.pdf
Orr, J. (2018). Developing a campus academic integrity education seminar. J Acad Ethics 16(3), 195–209. https://doi.org/10.1007/s10805-018-9304-7
Quinn, M. (2011). Introduction to Ethics. Ethics for an Information Age. 4th Ed. Ch 2. 53–108. Pearson. UK
Resnik. (2020). What is ethics in Research & why is it Important? Available Online. URL: https://www.niehs.nih.gov/research/resources/bioethics/whatis/index.cfm
Schnyder S, Starring H, Fury M, Mora A, Leonardi C, Dasa V (2018) The formation of a medical student research committee and its impact on involvement in departmental research. Med Educ Online 23(1):1. https://doi.org/10.1080/10872981.2018.1424449
Seymour E, Hunter AB, Laursen SL, DeAntoni T (2004) Establishing the benefits of research experiences for undergraduates in the sciences: first findings from a three-year study. Sci Educ 88(4):493–534. https://doi.org/10.1002/sce.10131
Shamoo AE, Irving DN (1993) Accountability in research using persons with mental illness. Account Res 3(1):1–17. https://doi.org/10.1080/08989629308573826
Shaw, S., Boynton, PM., and Greenhalgh, T. (2005). Research governance: where did it come from, what does it mean? Research governance framework for health and social care, 2nd ed. London: Department of Health. https://doi.org/10.1258/jrsm.98.11.496 , 98, 11, 496, 502
Book Google Scholar
Speight, JG. (2016) Ethics in the university |DOI: https://doi.org/10.1002/9781119346449 scrivener publishing LLC
Stephenson GK, Jones GA, Fick E, Begin-Caouette O, Taiyeb A, Metcalfe A (2020) What’s the protocol? Canadian university research ethics boards and variations in implementing tri-Council policy. Can J Higher Educ 50(1)1): 68–81
Surbhi, S. (2015). Difference between morals and ethics [weblog]. March 25, 2015. Retrieved February 14, 2021. Available Online. URL: http://keydifferences.com/difference-between-morals-and-ethics.html
The Belmont Report (1979). Ethical Principles and Guidelines for the Protection of Human Subjects of Research. The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. Retrieved February 14, 2021. Available online. URL: https://www.hhs.gov/ohrp/sites/default/files/the-belmont-report-508c_FINAL.pdf
The Singapore Statement on Research Integrity. (2020). Nicholas Steneck and Tony Mayer, Co-chairs, 2nd World Conference on Research Integrity; Melissa Anderson, Chair, Organizing Committee, 3rd World Conference on Research Integrity. Retrieved February 14, 2021. Available online. URL: https://wcrif.org/documents/327-singapore-statement-a4size/file
Warwick K (2003) Cyborg morals, cyborg values, cyborg ethics. Ethics Inf Technol 5(3):131–137. https://doi.org/10.1023/B:ETIN.0000006870.65865.cf
Weindling P (2001) The origins of informed consent: the international scientific commission on medical war crimes, and the Nuremberg code. Bull Hist Med 75(1):37–71. https://doi.org/10.1353/bhm.2001.0049
WHO. (2009). Research ethics committees Basic concepts for capacity-building. Retrieved February 14, 2021. Available online. URL: https://www.who.int/ethics/Ethics_basic_concepts_ENG.pdf
WHO. (2021). Chronological list of publications. Retrieved February 14, 2021. Available online. URL: https://www.who.int/ethics/publications/year/en/
Willison, J. and O’Regan, K. (2007). Commonly known, commonly not known, totally unknown: a framework for students becoming researchers. High Educ Res Dev 26(4). 393–409. https://doi.org/10.1080/07294360701658609
Žukauskas P, Vveinhardt J, and Andriukaitienė R. (2018). Research Ethics In book: Management Culture and Corporate Social Responsibility Eds Jolita Vveinhardt IntechOpenEditors DOI: https://doi.org/10.5772/intechopen.70629 , 2018
Download references
Acknowledgements
Authors wish to thank the organising committee of the 5th international conference named plagiarism across Europe and beyond, in Vilnius, Lithuania for accepting this paper to be presented in the conference.
Not applicable as this is an independent study, which is not funded by any internal or external bodies.
Author information
Authors and affiliations.
School of Human Sciences, University of Derby, DE22 1, Derby, GB, UK
Shivadas Sivasubramaniam
Department of Informatics, Mendel University in Brno, Zemědělská, 1665, Brno, Czechia
Dita Henek Dlabolová
Centre for Academic Integrity in the UAE, Faculty of Engineering & Information Sciences, University of Wollongong in Dubai, Dubai, UAE
Veronika Kralikova & Zeenath Reza Khan
You can also search for this author in PubMed Google Scholar
Contributions
The manuscript was entirely written by the corresponding author with contributions from co-authors who have equally contributed to presentation of this paper in the 5th international conference named plagiarism across Europe and beyond, in Vilnius, Lithuania. Authors have equally contributed for the information collection, which were then summarised as narrative explanations by the Corresponding author and Dr. Zeenath Reza Khan. Then checked and verified by Dr. Dlabolova and Ms. Králíková. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Shivadas Sivasubramaniam .
Ethics declarations
Competing interests.
We can also confirm that there are no potential competing interest with other organisations.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Sivasubramaniam, S., Dlabolová, D.H., Kralikova, V. et al. Assisting you to advance with ethics in research: an introduction to ethical governance and application procedures. Int J Educ Integr 17 , 14 (2021). https://doi.org/10.1007/s40979-021-00078-6
Download citation
Received : 17 July 2020
Accepted : 25 April 2021
Published : 13 July 2021
DOI : https://doi.org/10.1007/s40979-021-00078-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Higher education
- Ethical codes
- Ethics committee
- Post-secondary education
- Institutional policies
- Research ethics
International Journal for Educational Integrity
ISSN: 1833-2595
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- CORRESPONDENCE
- 02 July 2024
To regain lost public trust, incorporate research ethics into graduate training
- Lixin Wang 0
Indiana University Indianapolis, Indianapolis, Indiana, USA.
You can also search for this author in PubMed Google Scholar
Public trust in science in the United States, although high relative to trust in other institutions, has been declining ( A. Lupia et al. Proc. Natl Acad. Sci. USA 121 , e2319488121; 2024 ). Increasing reports of unethical behaviour, such as the recent scandal in high-temperature superconductivity (see Nature https://doi.org/mmz2; 2024 ), risk compounding this.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Nature 631 , 28 (2024)
doi: https://doi.org/10.1038/d41586-024-02131-z
Competing Interests
The author declares no competing interests.
Related Articles
See more letters to the editor
- Scientific community
- Research management
Boycotting academics in Israel is counterproductive
Correspondence 18 JUN 24

Why museums should repatriate fossils
Comment 18 JUN 24
Science profits most when people of faith feel equally welcomed
Correspondence 11 JUN 24
Unchanged power dynamics still block progress for under-represented groups in academia
Correspondence 02 JUL 24

What it means to be a successful male academic
Career Column 26 JUN 24

The strategy behind one of the most successful labs in the world
Comment 26 JUN 24

Is your research a trade secret? South Korean data-sharing case is a wake-up call
World View 03 JUL 24

How Rwandan paediatrician Agnes Binagwaho fights racial stereotypes in global health
Career Q&A 02 JUL 24
Junior Group Leader
The Imagine Institute is a leading European research centre dedicated to genetic diseases, with the primary objective to better understand and trea...
Paris, Ile-de-France (FR)
Imagine Institute
[DGIST] 2024 Tenure-Track Faculty Public Invitation
South Korea (KR)
Postdoctoral / Research Scientist / Research Assistant positions in Molecular Immunology
Postdoctoral / Research Scientist / Research Assistant positions in Molecular Immunology / Cancer Immunology
Dallas, Texas (US)
The University of Texas Southwestern Medical Center (UT Southwestern Medical Center)
Alzheimer's Disease (AD) Researcher/Associate Researcher
Xiaoliang Sunney XIE’s Group is recruiting researchers specializing in Alzheimer's disease (AD).
Beijing, China
Changping Laboratory
Osaka University Immunology Frontier Research Center Postdoctoral Researcher
IFReC, Osaka University in Japan offers Advanced Postdoc Positions for Immunology, Cell Biology, Bioinformatics and Bioimaging.
Suita Campus, Osaka University in Osaka, Japan
Immunology Frontier Research Center, Osaka University
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
National Institute of Environmental Health Sciences
Your environment. your health., what is ethics in research & why is it important, by david b. resnik, j.d., ph.d..
December 23, 2020
The ideas and opinions expressed in this essay are the author’s own and do not necessarily represent those of the NIH, NIEHS, or US government.

When most people think of ethics (or morals), they think of rules for distinguishing between right and wrong, such as the Golden Rule ("Do unto others as you would have them do unto you"), a code of professional conduct like the Hippocratic Oath ("First of all, do no harm"), a religious creed like the Ten Commandments ("Thou Shalt not kill..."), or a wise aphorisms like the sayings of Confucius. This is the most common way of defining "ethics": norms for conduct that distinguish between acceptable and unacceptable behavior.
Most people learn ethical norms at home, at school, in church, or in other social settings. Although most people acquire their sense of right and wrong during childhood, moral development occurs throughout life and human beings pass through different stages of growth as they mature. Ethical norms are so ubiquitous that one might be tempted to regard them as simple commonsense. On the other hand, if morality were nothing more than commonsense, then why are there so many ethical disputes and issues in our society?
Alternatives to Animal Testing
Alternative test methods are methods that replace, reduce, or refine animal use in research and testing
Learn more about Environmental science Basics
One plausible explanation of these disagreements is that all people recognize some common ethical norms but interpret, apply, and balance them in different ways in light of their own values and life experiences. For example, two people could agree that murder is wrong but disagree about the morality of abortion because they have different understandings of what it means to be a human being.
Most societies also have legal rules that govern behavior, but ethical norms tend to be broader and more informal than laws. Although most societies use laws to enforce widely accepted moral standards and ethical and legal rules use similar concepts, ethics and law are not the same. An action may be legal but unethical or illegal but ethical. We can also use ethical concepts and principles to criticize, evaluate, propose, or interpret laws. Indeed, in the last century, many social reformers have urged citizens to disobey laws they regarded as immoral or unjust laws. Peaceful civil disobedience is an ethical way of protesting laws or expressing political viewpoints.
Another way of defining 'ethics' focuses on the disciplines that study standards of conduct, such as philosophy, theology, law, psychology, or sociology. For example, a "medical ethicist" is someone who studies ethical standards in medicine. One may also define ethics as a method, procedure, or perspective for deciding how to act and for analyzing complex problems and issues. For instance, in considering a complex issue like global warming , one may take an economic, ecological, political, or ethical perspective on the problem. While an economist might examine the cost and benefits of various policies related to global warming, an environmental ethicist could examine the ethical values and principles at stake.
See ethics in practice at NIEHS
Read latest updates in our monthly Global Environmental Health Newsletter

Many different disciplines, institutions , and professions have standards for behavior that suit their particular aims and goals. These standards also help members of the discipline to coordinate their actions or activities and to establish the public's trust of the discipline. For instance, ethical standards govern conduct in medicine, law, engineering, and business. Ethical norms also serve the aims or goals of research and apply to people who conduct scientific research or other scholarly or creative activities. There is even a specialized discipline, research ethics, which studies these norms. See Glossary of Commonly Used Terms in Research Ethics and Research Ethics Timeline .
There are several reasons why it is important to adhere to ethical norms in research. First, norms promote the aims of research , such as knowledge, truth, and avoidance of error. For example, prohibitions against fabricating , falsifying, or misrepresenting research data promote the truth and minimize error.
Join an NIEHS Study
See how we put research Ethics to practice.
Visit Joinastudy.niehs.nih.gov to see the various studies NIEHS perform.

Second, since research often involves a great deal of cooperation and coordination among many different people in different disciplines and institutions, ethical standards promote the values that are essential to collaborative work , such as trust, accountability, mutual respect, and fairness. For example, many ethical norms in research, such as guidelines for authorship , copyright and patenting policies , data sharing policies, and confidentiality rules in peer review, are designed to protect intellectual property interests while encouraging collaboration. Most researchers want to receive credit for their contributions and do not want to have their ideas stolen or disclosed prematurely.
Third, many of the ethical norms help to ensure that researchers can be held accountable to the public . For instance, federal policies on research misconduct, conflicts of interest, the human subjects protections, and animal care and use are necessary in order to make sure that researchers who are funded by public money can be held accountable to the public.
Fourth, ethical norms in research also help to build public support for research. People are more likely to fund a research project if they can trust the quality and integrity of research.
Finally, many of the norms of research promote a variety of other important moral and social values , such as social responsibility, human rights, animal welfare, compliance with the law, and public health and safety. Ethical lapses in research can significantly harm human and animal subjects, students, and the public. For example, a researcher who fabricates data in a clinical trial may harm or even kill patients, and a researcher who fails to abide by regulations and guidelines relating to radiation or biological safety may jeopardize his health and safety or the health and safety of staff and students.
Codes and Policies for Research Ethics
Given the importance of ethics for the conduct of research, it should come as no surprise that many different professional associations, government agencies, and universities have adopted specific codes, rules, and policies relating to research ethics. Many government agencies have ethics rules for funded researchers.
- National Institutes of Health (NIH)
- National Science Foundation (NSF)
- Food and Drug Administration (FDA)
- Environmental Protection Agency (EPA)
- US Department of Agriculture (USDA)
- Singapore Statement on Research Integrity
- American Chemical Society, The Chemist Professional’s Code of Conduct
- Code of Ethics (American Society for Clinical Laboratory Science)
- American Psychological Association, Ethical Principles of Psychologists and Code of Conduct
- Statement on Professional Ethics (American Association of University Professors)
- Nuremberg Code
- World Medical Association's Declaration of Helsinki
Ethical Principles
The following is a rough and general summary of some ethical principles that various codes address*:

Strive for honesty in all scientific communications. Honestly report data, results, methods and procedures, and publication status. Do not fabricate, falsify, or misrepresent data. Do not deceive colleagues, research sponsors, or the public.

Objectivity
Strive to avoid bias in experimental design, data analysis, data interpretation, peer review, personnel decisions, grant writing, expert testimony, and other aspects of research where objectivity is expected or required. Avoid or minimize bias or self-deception. Disclose personal or financial interests that may affect research.

Keep your promises and agreements; act with sincerity; strive for consistency of thought and action.

Carefulness
Avoid careless errors and negligence; carefully and critically examine your own work and the work of your peers. Keep good records of research activities, such as data collection, research design, and correspondence with agencies or journals.

Share data, results, ideas, tools, resources. Be open to criticism and new ideas.
Transparency
Disclose methods, materials, assumptions, analyses, and other information needed to evaluate your research.

Accountability
Take responsibility for your part in research and be prepared to give an account (i.e. an explanation or justification) of what you did on a research project and why.

Intellectual Property
Honor patents, copyrights, and other forms of intellectual property. Do not use unpublished data, methods, or results without permission. Give proper acknowledgement or credit for all contributions to research. Never plagiarize.

Confidentiality
Protect confidential communications, such as papers or grants submitted for publication, personnel records, trade or military secrets, and patient records.

Responsible Publication
Publish in order to advance research and scholarship, not to advance just your own career. Avoid wasteful and duplicative publication.

Responsible Mentoring
Help to educate, mentor, and advise students. Promote their welfare and allow them to make their own decisions.

Respect for Colleagues
Respect your colleagues and treat them fairly.

Social Responsibility
Strive to promote social good and prevent or mitigate social harms through research, public education, and advocacy.

Non-Discrimination
Avoid discrimination against colleagues or students on the basis of sex, race, ethnicity, or other factors not related to scientific competence and integrity.

Maintain and improve your own professional competence and expertise through lifelong education and learning; take steps to promote competence in science as a whole.

Know and obey relevant laws and institutional and governmental policies.

Animal Care
Show proper respect and care for animals when using them in research. Do not conduct unnecessary or poorly designed animal experiments.

Human Subjects protection
When conducting research on human subjects, minimize harms and risks and maximize benefits; respect human dignity, privacy, and autonomy; take special precautions with vulnerable populations; and strive to distribute the benefits and burdens of research fairly.
* Adapted from Shamoo A and Resnik D. 2015. Responsible Conduct of Research, 3rd ed. (New York: Oxford University Press).
Ethical Decision Making in Research
Although codes, policies, and principles are very important and useful, like any set of rules, they do not cover every situation, they often conflict, and they require interpretation. It is therefore important for researchers to learn how to interpret, assess, and apply various research rules and how to make decisions and act ethically in various situations. The vast majority of decisions involve the straightforward application of ethical rules. For example, consider the following case:
The research protocol for a study of a drug on hypertension requires the administration of the drug at different doses to 50 laboratory mice, with chemical and behavioral tests to determine toxic effects. Tom has almost finished the experiment for Dr. Q. He has only 5 mice left to test. However, he really wants to finish his work in time to go to Florida on spring break with his friends, who are leaving tonight. He has injected the drug in all 50 mice but has not completed all of the tests. He therefore decides to extrapolate from the 45 completed results to produce the 5 additional results.
Many different research ethics policies would hold that Tom has acted unethically by fabricating data. If this study were sponsored by a federal agency, such as the NIH, his actions would constitute a form of research misconduct , which the government defines as "fabrication, falsification, or plagiarism" (or FFP). Actions that nearly all researchers classify as unethical are viewed as misconduct. It is important to remember, however, that misconduct occurs only when researchers intend to deceive : honest errors related to sloppiness, poor record keeping, miscalculations, bias, self-deception, and even negligence do not constitute misconduct. Also, reasonable disagreements about research methods, procedures, and interpretations do not constitute research misconduct. Consider the following case:
Dr. T has just discovered a mathematical error in his paper that has been accepted for publication in a journal. The error does not affect the overall results of his research, but it is potentially misleading. The journal has just gone to press, so it is too late to catch the error before it appears in print. In order to avoid embarrassment, Dr. T decides to ignore the error.
Dr. T's error is not misconduct nor is his decision to take no action to correct the error. Most researchers, as well as many different policies and codes would say that Dr. T should tell the journal (and any coauthors) about the error and consider publishing a correction or errata. Failing to publish a correction would be unethical because it would violate norms relating to honesty and objectivity in research.
There are many other activities that the government does not define as "misconduct" but which are still regarded by most researchers as unethical. These are sometimes referred to as " other deviations " from acceptable research practices and include:
- Publishing the same paper in two different journals without telling the editors
- Submitting the same paper to different journals without telling the editors
- Not informing a collaborator of your intent to file a patent in order to make sure that you are the sole inventor
- Including a colleague as an author on a paper in return for a favor even though the colleague did not make a serious contribution to the paper
- Discussing with your colleagues confidential data from a paper that you are reviewing for a journal
- Using data, ideas, or methods you learn about while reviewing a grant or a papers without permission
- Trimming outliers from a data set without discussing your reasons in paper
- Using an inappropriate statistical technique in order to enhance the significance of your research
- Bypassing the peer review process and announcing your results through a press conference without giving peers adequate information to review your work
- Conducting a review of the literature that fails to acknowledge the contributions of other people in the field or relevant prior work
- Stretching the truth on a grant application in order to convince reviewers that your project will make a significant contribution to the field
- Stretching the truth on a job application or curriculum vita
- Giving the same research project to two graduate students in order to see who can do it the fastest
- Overworking, neglecting, or exploiting graduate or post-doctoral students
- Failing to keep good research records
- Failing to maintain research data for a reasonable period of time
- Making derogatory comments and personal attacks in your review of author's submission
- Promising a student a better grade for sexual favors
- Using a racist epithet in the laboratory
- Making significant deviations from the research protocol approved by your institution's Animal Care and Use Committee or Institutional Review Board for Human Subjects Research without telling the committee or the board
- Not reporting an adverse event in a human research experiment
- Wasting animals in research
- Exposing students and staff to biological risks in violation of your institution's biosafety rules
- Sabotaging someone's work
- Stealing supplies, books, or data
- Rigging an experiment so you know how it will turn out
- Making unauthorized copies of data, papers, or computer programs
- Owning over $10,000 in stock in a company that sponsors your research and not disclosing this financial interest
- Deliberately overestimating the clinical significance of a new drug in order to obtain economic benefits
These actions would be regarded as unethical by most scientists and some might even be illegal in some cases. Most of these would also violate different professional ethics codes or institutional policies. However, they do not fall into the narrow category of actions that the government classifies as research misconduct. Indeed, there has been considerable debate about the definition of "research misconduct" and many researchers and policy makers are not satisfied with the government's narrow definition that focuses on FFP. However, given the huge list of potential offenses that might fall into the category "other serious deviations," and the practical problems with defining and policing these other deviations, it is understandable why government officials have chosen to limit their focus.
Finally, situations frequently arise in research in which different people disagree about the proper course of action and there is no broad consensus about what should be done. In these situations, there may be good arguments on both sides of the issue and different ethical principles may conflict. These situations create difficult decisions for research known as ethical or moral dilemmas . Consider the following case:
Dr. Wexford is the principal investigator of a large, epidemiological study on the health of 10,000 agricultural workers. She has an impressive dataset that includes information on demographics, environmental exposures, diet, genetics, and various disease outcomes such as cancer, Parkinson’s disease (PD), and ALS. She has just published a paper on the relationship between pesticide exposure and PD in a prestigious journal. She is planning to publish many other papers from her dataset. She receives a request from another research team that wants access to her complete dataset. They are interested in examining the relationship between pesticide exposures and skin cancer. Dr. Wexford was planning to conduct a study on this topic.
Dr. Wexford faces a difficult choice. On the one hand, the ethical norm of openness obliges her to share data with the other research team. Her funding agency may also have rules that obligate her to share data. On the other hand, if she shares data with the other team, they may publish results that she was planning to publish, thus depriving her (and her team) of recognition and priority. It seems that there are good arguments on both sides of this issue and Dr. Wexford needs to take some time to think about what she should do. One possible option is to share data, provided that the investigators sign a data use agreement. The agreement could define allowable uses of the data, publication plans, authorship, etc. Another option would be to offer to collaborate with the researchers.
The following are some step that researchers, such as Dr. Wexford, can take to deal with ethical dilemmas in research:
What is the problem or issue?
It is always important to get a clear statement of the problem. In this case, the issue is whether to share information with the other research team.
What is the relevant information?
Many bad decisions are made as a result of poor information. To know what to do, Dr. Wexford needs to have more information concerning such matters as university or funding agency or journal policies that may apply to this situation, the team's intellectual property interests, the possibility of negotiating some kind of agreement with the other team, whether the other team also has some information it is willing to share, the impact of the potential publications, etc.

What are the different options?
People may fail to see different options due to a limited imagination, bias, ignorance, or fear. In this case, there may be other choices besides 'share' or 'don't share,' such as 'negotiate an agreement' or 'offer to collaborate with the researchers.'
How do ethical codes or policies as well as legal rules apply to these different options?
The university or funding agency may have policies on data management that apply to this case. Broader ethical rules, such as openness and respect for credit and intellectual property, may also apply to this case. Laws relating to intellectual property may be relevant.
Are there any people who can offer ethical advice?
It may be useful to seek advice from a colleague, a senior researcher, your department chair, an ethics or compliance officer, or anyone else you can trust. In the case, Dr. Wexford might want to talk to her supervisor and research team before making a decision.
After considering these questions, a person facing an ethical dilemma may decide to ask more questions, gather more information, explore different options, or consider other ethical rules. However, at some point he or she will have to make a decision and then take action. Ideally, a person who makes a decision in an ethical dilemma should be able to justify his or her decision to himself or herself, as well as colleagues, administrators, and other people who might be affected by the decision. He or she should be able to articulate reasons for his or her conduct and should consider the following questions in order to explain how he or she arrived at his or her decision:
- Which choice will probably have the best overall consequences for science and society?
- Which choice could stand up to further publicity and scrutiny?
- Which choice could you not live with?
- Think of the wisest person you know. What would he or she do in this situation?
- Which choice would be the most just, fair, or responsible?
After considering all of these questions, one still might find it difficult to decide what to do. If this is the case, then it may be appropriate to consider others ways of making the decision, such as going with a gut feeling or intuition, seeking guidance through prayer or meditation, or even flipping a coin. Endorsing these methods in this context need not imply that ethical decisions are irrational, however. The main point is that human reasoning plays a pivotal role in ethical decision-making but there are limits to its ability to solve all ethical dilemmas in a finite amount of time.
Promoting Ethical Conduct in Science

Do U.S. research institutions meet or exceed federal mandates for instruction in responsible conduct of research? A national survey

Read about U.S. research instutuins follow federal manadates for ethics in research
Learn more about NIEHS Research
Most academic institutions in the US require undergraduate, graduate, or postgraduate students to have some education in the responsible conduct of research (RCR) . The NIH and NSF have both mandated training in research ethics for students and trainees. Many academic institutions outside of the US have also developed educational curricula in research ethics
Those of you who are taking or have taken courses in research ethics may be wondering why you are required to have education in research ethics. You may believe that you are highly ethical and know the difference between right and wrong. You would never fabricate or falsify data or plagiarize. Indeed, you also may believe that most of your colleagues are highly ethical and that there is no ethics problem in research..
If you feel this way, relax. No one is accusing you of acting unethically. Indeed, the evidence produced so far shows that misconduct is a very rare occurrence in research, although there is considerable variation among various estimates. The rate of misconduct has been estimated to be as low as 0.01% of researchers per year (based on confirmed cases of misconduct in federally funded research) to as high as 1% of researchers per year (based on self-reports of misconduct on anonymous surveys). See Shamoo and Resnik (2015), cited above.
Clearly, it would be useful to have more data on this topic, but so far there is no evidence that science has become ethically corrupt, despite some highly publicized scandals. Even if misconduct is only a rare occurrence, it can still have a tremendous impact on science and society because it can compromise the integrity of research, erode the public’s trust in science, and waste time and resources. Will education in research ethics help reduce the rate of misconduct in science? It is too early to tell. The answer to this question depends, in part, on how one understands the causes of misconduct. There are two main theories about why researchers commit misconduct. According to the "bad apple" theory, most scientists are highly ethical. Only researchers who are morally corrupt, economically desperate, or psychologically disturbed commit misconduct. Moreover, only a fool would commit misconduct because science's peer review system and self-correcting mechanisms will eventually catch those who try to cheat the system. In any case, a course in research ethics will have little impact on "bad apples," one might argue.
According to the "stressful" or "imperfect" environment theory, misconduct occurs because various institutional pressures, incentives, and constraints encourage people to commit misconduct, such as pressures to publish or obtain grants or contracts, career ambitions, the pursuit of profit or fame, poor supervision of students and trainees, and poor oversight of researchers (see Shamoo and Resnik 2015). Moreover, defenders of the stressful environment theory point out that science's peer review system is far from perfect and that it is relatively easy to cheat the system. Erroneous or fraudulent research often enters the public record without being detected for years. Misconduct probably results from environmental and individual causes, i.e. when people who are morally weak, ignorant, or insensitive are placed in stressful or imperfect environments. In any case, a course in research ethics can be useful in helping to prevent deviations from norms even if it does not prevent misconduct. Education in research ethics is can help people get a better understanding of ethical standards, policies, and issues and improve ethical judgment and decision making. Many of the deviations that occur in research may occur because researchers simply do not know or have never thought seriously about some of the ethical norms of research. For example, some unethical authorship practices probably reflect traditions and practices that have not been questioned seriously until recently. If the director of a lab is named as an author on every paper that comes from his lab, even if he does not make a significant contribution, what could be wrong with that? That's just the way it's done, one might argue. Another example where there may be some ignorance or mistaken traditions is conflicts of interest in research. A researcher may think that a "normal" or "traditional" financial relationship, such as accepting stock or a consulting fee from a drug company that sponsors her research, raises no serious ethical issues. Or perhaps a university administrator sees no ethical problem in taking a large gift with strings attached from a pharmaceutical company. Maybe a physician thinks that it is perfectly appropriate to receive a $300 finder’s fee for referring patients into a clinical trial.
If "deviations" from ethical conduct occur in research as a result of ignorance or a failure to reflect critically on problematic traditions, then a course in research ethics may help reduce the rate of serious deviations by improving the researcher's understanding of ethics and by sensitizing him or her to the issues.
Finally, education in research ethics should be able to help researchers grapple with the ethical dilemmas they are likely to encounter by introducing them to important concepts, tools, principles, and methods that can be useful in resolving these dilemmas. Scientists must deal with a number of different controversial topics, such as human embryonic stem cell research, cloning, genetic engineering, and research involving animal or human subjects, which require ethical reflection and deliberation.
- Privacy Policy

Home » Ethical Considerations – Types, Examples and Writing Guide
Ethical Considerations – Types, Examples and Writing Guide
Table of Contents

Ethical Considerations
Ethical considerations in research refer to the principles and guidelines that researchers must follow to ensure that their studies are conducted in an ethical and responsible manner. These considerations are designed to protect the rights, safety, and well-being of research participants, as well as the integrity and credibility of the research itself
Some of the key ethical considerations in research include:
- Informed consent: Researchers must obtain informed consent from study participants, which means they must inform participants about the study’s purpose, procedures, risks, benefits, and their right to withdraw at any time.
- Privacy and confidentiality : Researchers must ensure that participants’ privacy and confidentiality are protected. This means that personal information should be kept confidential and not shared without the participant’s consent.
- Harm reduction : Researchers must ensure that the study does not harm the participants physically or psychologically. They must take steps to minimize the risks associated with the study.
- Fairness and equity : Researchers must ensure that the study does not discriminate against any particular group or individual. They should treat all participants equally and fairly.
- Use of deception: Researchers must use deception only if it is necessary to achieve the study’s objectives. They must inform participants of the deception as soon as possible.
- Use of vulnerable populations : Researchers must be especially cautious when working with vulnerable populations, such as children, pregnant women, prisoners, and individuals with cognitive or intellectual disabilities.
- Conflict of interest : Researchers must disclose any potential conflicts of interest that may affect the study’s integrity. This includes financial or personal relationships that could influence the study’s results.
- Data manipulation: Researchers must not manipulate data to support a particular hypothesis or agenda. They should report the results of the study objectively, even if the findings are not consistent with their expectations.
- Intellectual property: Researchers must respect intellectual property rights and give credit to previous studies and research.
- Cultural sensitivity : Researchers must be sensitive to the cultural norms and beliefs of the participants. They should avoid imposing their values and beliefs on the participants and should be respectful of their cultural practices.
Types of Ethical Considerations
Types of Ethical Considerations are as follows:
Research Ethics:
This includes ethical principles and guidelines that govern research involving human or animal subjects, ensuring that the research is conducted in an ethical and responsible manner.
Business Ethics :
This refers to ethical principles and standards that guide business practices and decision-making, such as transparency, honesty, fairness, and social responsibility.
Medical Ethics :
This refers to ethical principles and standards that govern the practice of medicine, including the duty to protect patient autonomy, informed consent, confidentiality, and non-maleficence.
Environmental Ethics :
This involves ethical principles and values that guide our interactions with the natural world, including the obligation to protect the environment, minimize harm, and promote sustainability.
Legal Ethics
This involves ethical principles and standards that guide the conduct of legal professionals, including issues such as confidentiality, conflicts of interest, and professional competence.
Social Ethics
This involves ethical principles and values that guide our interactions with other individuals and society as a whole, including issues such as justice, fairness, and human rights.
Information Ethics
This involves ethical principles and values that govern the use and dissemination of information, including issues such as privacy, accuracy, and intellectual property.
Cultural Ethics
This involves ethical principles and values that govern the relationship between different cultures and communities, including issues such as respect for diversity, cultural sensitivity, and inclusivity.
Technological Ethics
This refers to ethical principles and guidelines that govern the development, use, and impact of technology, including issues such as privacy, security, and social responsibility.
Journalism Ethics
This involves ethical principles and standards that guide the practice of journalism, including issues such as accuracy, fairness, and the public interest.
Educational Ethics
This refers to ethical principles and standards that guide the practice of education, including issues such as academic integrity, fairness, and respect for diversity.
Political Ethics
This involves ethical principles and values that guide political decision-making and behavior, including issues such as accountability, transparency, and the protection of civil liberties.
Professional Ethics
This refers to ethical principles and standards that guide the conduct of professionals in various fields, including issues such as honesty, integrity, and competence.
Personal Ethics
This involves ethical principles and values that guide individual behavior and decision-making, including issues such as personal responsibility, honesty, and respect for others.
Global Ethics
This involves ethical principles and values that guide our interactions with other nations and the global community, including issues such as human rights, environmental protection, and social justice.
Applications of Ethical Considerations
Ethical considerations are important in many areas of society, including medicine, business, law, and technology. Here are some specific applications of ethical considerations:
- Medical research : Ethical considerations are crucial in medical research, particularly when human subjects are involved. Researchers must ensure that their studies are conducted in a way that does not harm participants and that participants give informed consent before participating.
- Business practices: Ethical considerations are also important in business, where companies must make decisions that are socially responsible and avoid activities that are harmful to society. For example, companies must ensure that their products are safe for consumers and that they do not engage in exploitative labor practices.
- Environmental protection: Ethical considerations play a crucial role in environmental protection, as companies and governments must weigh the benefits of economic development against the potential harm to the environment. Decisions about land use, resource allocation, and pollution must be made in an ethical manner that takes into account the long-term consequences for the planet and future generations.
- Technology development : As technology continues to advance rapidly, ethical considerations become increasingly important in areas such as artificial intelligence, robotics, and genetic engineering. Developers must ensure that their creations do not harm humans or the environment and that they are developed in a way that is fair and equitable.
- Legal system : The legal system relies on ethical considerations to ensure that justice is served and that individuals are treated fairly. Lawyers and judges must abide by ethical standards to maintain the integrity of the legal system and to protect the rights of all individuals involved.
Examples of Ethical Considerations
Here are a few examples of ethical considerations in different contexts:
- In healthcare : A doctor must ensure that they provide the best possible care to their patients and avoid causing them harm. They must respect the autonomy of their patients, and obtain informed consent before administering any treatment or procedure. They must also ensure that they maintain patient confidentiality and avoid any conflicts of interest.
- In the workplace: An employer must ensure that they treat their employees fairly and with respect, provide them with a safe working environment, and pay them a fair wage. They must also avoid any discrimination based on race, gender, religion, or any other characteristic protected by law.
- In the media : Journalists must ensure that they report the news accurately and without bias. They must respect the privacy of individuals and avoid causing harm or distress. They must also be transparent about their sources and avoid any conflicts of interest.
- In research: Researchers must ensure that they conduct their studies ethically and with integrity. They must obtain informed consent from participants, protect their privacy, and avoid any harm or discomfort. They must also ensure that their findings are reported accurately and without bias.
- In personal relationships : People must ensure that they treat others with respect and kindness, and avoid causing harm or distress. They must respect the autonomy of others and avoid any actions that would be considered unethical, such as lying or cheating. They must also respect the confidentiality of others and maintain their privacy.
How to Write Ethical Considerations
When writing about research involving human subjects or animals, it is essential to include ethical considerations to ensure that the study is conducted in a manner that is morally responsible and in accordance with professional standards. Here are some steps to help you write ethical considerations:
- Describe the ethical principles: Start by explaining the ethical principles that will guide the research. These could include principles such as respect for persons, beneficence, and justice.
- Discuss informed consent : Informed consent is a critical ethical consideration when conducting research. Explain how you will obtain informed consent from participants, including how you will explain the purpose of the study, potential risks and benefits, and how you will protect their privacy.
- Address confidentiality : Describe how you will protect the confidentiality of the participants’ personal information and data, including any measures you will take to ensure that the data is kept secure and confidential.
- Consider potential risks and benefits : Describe any potential risks or harms to participants that could result from the study and how you will minimize those risks. Also, discuss the potential benefits of the study, both to the participants and to society.
- Discuss the use of animals : If the research involves the use of animals, address the ethical considerations related to animal welfare. Explain how you will minimize any potential harm to the animals and ensure that they are treated ethically.
- Mention the ethical approval : Finally, it’s essential to acknowledge that the research has received ethical approval from the relevant institutional review board or ethics committee. State the name of the committee, the date of approval, and any specific conditions or requirements that were imposed.
When to Write Ethical Considerations
Ethical considerations should be written whenever research involves human subjects or has the potential to impact human beings, animals, or the environment in some way. Ethical considerations are also important when research involves sensitive topics, such as mental health, sexuality, or religion.
In general, ethical considerations should be an integral part of any research project, regardless of the field or subject matter. This means that they should be considered at every stage of the research process, from the initial planning and design phase to data collection, analysis, and dissemination.
Ethical considerations should also be written in accordance with the guidelines and standards set by the relevant regulatory bodies and professional associations. These guidelines may vary depending on the discipline, so it is important to be familiar with the specific requirements of your field.
Purpose of Ethical Considerations
Ethical considerations are an essential aspect of many areas of life, including business, healthcare, research, and social interactions. The primary purposes of ethical considerations are:
- Protection of human rights: Ethical considerations help ensure that people’s rights are respected and protected. This includes respecting their autonomy, ensuring their privacy is respected, and ensuring that they are not subjected to harm or exploitation.
- Promoting fairness and justice: Ethical considerations help ensure that people are treated fairly and justly, without discrimination or bias. This includes ensuring that everyone has equal access to resources and opportunities, and that decisions are made based on merit rather than personal biases or prejudices.
- Promoting honesty and transparency : Ethical considerations help ensure that people are truthful and transparent in their actions and decisions. This includes being open and honest about conflicts of interest, disclosing potential risks, and communicating clearly with others.
- Maintaining public trust: Ethical considerations help maintain public trust in institutions and individuals. This is important for building and maintaining relationships with customers, patients, colleagues, and other stakeholders.
- Ensuring responsible conduct: Ethical considerations help ensure that people act responsibly and are accountable for their actions. This includes adhering to professional standards and codes of conduct, following laws and regulations, and avoiding behaviors that could harm others or damage the environment.
Advantages of Ethical Considerations
Here are some of the advantages of ethical considerations:
- Builds Trust : When individuals or organizations follow ethical considerations, it creates a sense of trust among stakeholders, including customers, clients, and employees. This trust can lead to stronger relationships and long-term loyalty.
- Reputation and Brand Image : Ethical considerations are often linked to a company’s brand image and reputation. By following ethical practices, a company can establish a positive image and reputation that can enhance its brand value.
- Avoids Legal Issues: Ethical considerations can help individuals and organizations avoid legal issues and penalties. By adhering to ethical principles, companies can reduce the risk of facing lawsuits, regulatory investigations, and fines.
- Increases Employee Retention and Motivation: Employees tend to be more satisfied and motivated when they work for an organization that values ethics. Companies that prioritize ethical considerations tend to have higher employee retention rates, leading to lower recruitment costs.
- Enhances Decision-making: Ethical considerations help individuals and organizations make better decisions. By considering the ethical implications of their actions, decision-makers can evaluate the potential consequences and choose the best course of action.
- Positive Impact on Society: Ethical considerations have a positive impact on society as a whole. By following ethical practices, companies can contribute to social and environmental causes, leading to a more sustainable and equitable society.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Data Analysis – Process, Methods and Types

Theoretical Framework – Types, Examples and...

Background of The Study – Examples and Writing...

References in Research – Types, Examples and...

Appendix in Research Paper – Examples and...

Limitations in Research – Types, Examples and...
share this!
June 25, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
A model of Collaborative Ethics to guide translational research from fundamental discoveries to real-world applications
by Benjamin Boettner, Harvard University
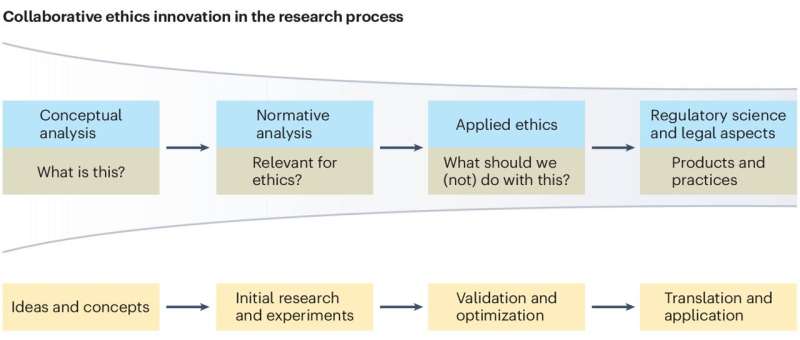
In sciences, disruptive research that is breaking new ground often raises new and not-yet-explored ethical questions. Although new scientific breakthroughs can have the power to change how we understand and live in the world, the ethical implications of technologies that will emerge based on these new insights can affect an emerging field's public acceptance and have moral implications for society at large. They can also impact the process of translating discoveries into real-world products, sometimes requiring new regulations.
Historically, ethicists—who form the branch of philosophy that is concerned with morality and studies of human conduct in relation to the self, others, and the natural world—were only given the opportunity to analyze transformative breakthroughs in the life sciences after-the-fact, when new scientific possibilities already start to impact the real world. This has often prevented the creation of crucial decision moments and course corrections before ethical controversies gained momentum and bioethicists specializing on medical, public health, animal, or environmental ethics were called upon.
"To overcome current limitations, it is crucial that the analysis process be initiated at the earliest stages when fundamental discoveries are made to clearly answer the question of what, for example, newly created biological constructs are . This first analysis is actually an explicitly philosophical one—only when it determines that an 'ethical threshold' is reached should a more ethics-focused analysis follow," said Wyss Institute Collaborative Ethics lead Jeantine Lunshof. "This analysis ensures that an ethical analysis is conducted when it is relevant and useful."
Lunshof, in her collaborative work with researchers at the Wyss Institute and Harvard Medical School (HMS) over more than 15 years, has advanced a generic model of "Collaborative Ethics." There is special need for frequent philosophical and ethical reassessment in organizations, such as the Wyss Institute, which develop new breakthrough technologies and translate them at an extremely fast pace.
To ensure this is most functional, "The Collaborative Ethics effort is also aligned with the Wyss Institute's Technology Innovation Funnel, which formalizes the journey of its cutting-edge technologies starting at the 'Idea Generation' stage when fundamental discoveries are made and first assessed for their translational potential," said Lunshof, who holds a Ph.D. and M.A. in Philosophy and Health Law. "But they can be applied to translational research in any academic environment."
Now, Lunshof and Julia Rijssenbeek, a Graduate Student at Wageningen University in the Netherlands who did a Ph.D. internship at the Wyss Institute, have published the Collaborative Ethics model in Nature Methods .
In the Wyss' Translation Innovation Funnel, potentially life-saving and changing technologies proceed through "Concept Refinement," "Technology Validation," "Technology Optimization" and "Commercialization" stages. This path is paralleled by steps the Collaborative Ethics model provides for investigating philosophically and ethically relevant implications and creating further actionable decision points.
For Collaborative Ethics to work effectively, it is essential that the early dialogue between researchers and ethicists continues through all stages of technology development, all the way to the creation of products that can be commercialized.
"It doesn't suffice that a philosopher or an ethicist occasionally reviews a research group's work as an outsider or audits it for a different department or organization. For a meaningful dialogue to happen, it is essential that they become actual members of project teams to facilitate meaningful reflections during the scientific process instead of when it has been concluded," said Rijssenbeek.
"This immensely facilitates the exchange of thoughts in both directions, philosophical and ethical innovation, as well as decision-making processes that enable responsible research and innovation towards life-changing technologies."
Lunshof's philosophical and ethical work at Harvard University began in 2006 in the lab of Wyss Institute Core Faculty member George Church, Ph.D., who at HMS pioneered the decoding and study of genomes, particularly that of the human genome, and the relatively young field of synthetic biology. Church is also the Robert Winthrop Professor of Genetics at HMS. As Church's collaborator, Lunshof became involved with the Personal Genome Project, which was begun the previous year and aimed to sequence and publicize the complete genomes and medical records of 100,000 volunteers.
Subsequently, as a Marie Sklodowska Curie International Fellow, her interest expanded into the new realities of genome engineering, the creation of chimeras from cells stemming from different organisms, and other disruptive scientific advances made in Church's lab. Her participation helped pave the way toward a more balanced public perception of the research.
Lunshof continued her work by focusing on other "hot topic" ethical areas, some of them closely related to research conceived at the Wyss Institute. She co-led the Brainstorm Project, which was part of the US National Institutes of Health BRAIN initiative neuroethics program, where she helped create an ethically recommendable path forward for neural or "brain organoids" as models for human brain development and brain disease. The Wyss Institute's CircaVent Project, led by Church, uses brain organoids to model neurological and psychiatric disorders, in the discovery of urgently needed drugs.
Another example of ethically complex technology is "biobots," tiny computer-designed living organisms with programmed behaviors built from animal or human cells. Developed by Michael Levin's group at the Wyss Institute and Tufts University in collaboration with roboticists and computer scientists at the University of Vermont, biobots do not fit into any traditional ethical category. They raise important questions regarding their identities and what the researchers' intentions and goals are. Levin is an Associate Faculty member at the Wyss Institute as well as Distinguished Professor and Vannevar Bush Chair at Tufts University's Department of Biology.
Lunshof and Rijssenbeek used these and other examples to illustrate how the Collaborative Ethics model has been successfully applied in practice. Being integrated with the research process through four defined steps, it first carries out a "Conceptual Analysis," by asking the question "what is it?"—in the case of biobots, are these novel life forms organisms, robots, or machines?
The second step, "Normative Analysis," marks the transition from general philosophy to ethics and drills into the question of whether certain research raises any ethical concerns.
The model's third step, "Applied Ethics," is similar to bioethicist's role, and applies ethical theories to assess the real-world impact of technology developments like, for example, the use of animals in research, the provenance of human biological specimens, questions of privacy and consent, the benefits and risks of new technologies such as genome editing for patients, and the justification of gain-of-function research that could lead to new antimicrobials but also result in enhanced pathogens. The question of whether a finding that can bring benefits but also carries risks of harm should be published at all to prevent any misuse is also considered here.
Finally, in the "Regulatory Science and Legal Aspects" step, researchers and ethicists guided by the Collaborative Ethics model team up with business development and technology transfer experts to analyze how a technology can be translated into real-world applications by a spin-off or through licensing by industry.
"Because science and technology development are progressing at an ever-higher speed, and are globalizing rapidly, real-time collaboration between researchers and ethicists becomes vital. It enables a learning curve and necessary adjustments in the research process, as well as the development of missing ethical approaches and the revision of ethics positions," said Lunshof, who was named a Fellow of The Kavli Foundation which supports the advancement of science and the increase of public understanding and support for scientists and their work, earlier this year.
"In their relentless pursuit of urgently needed solutions for patients and the environment, Wyss Institute researchers not only collaborate across disciplines with one another and with industrial partners scientifically, but they also make sure that they evaluate their research from a philosophical and ethical perspective—the Collaborative Ethics model is an invaluable guide to accomplish this," said Wyss Founding Director Donald Ingber, M.D., Ph.D., who is also the Judah Folkman Professor of Vascular Biology at HMS and Boston Children's Hospital, and Hansjörg Wyss Professor of Bioinspired Engineering at the Harvard John A. Paulson School of Engineering and Applied Sciences.
Journal information: Nature Methods
Provided by Harvard University
Explore further
Feedback to editors
Scientists discover way to 'grow' sub-nanometer sized transistors
2 hours ago

Scientists pinpoint strategies that could stop cats from scratching your furniture
7 hours ago

Two new species of Psilocybe mushrooms discovered in southern Africa
15 hours ago

UV radiation damage leads to ribosome roadblocks, causing early skin cell death
16 hours ago

Dual-laser approach could lower cost of high-resolution 3D printing

Novel method enhances size-controlled production of luminescent quantum dots

Cosmic simulation reveals how black holes grow and evolve
17 hours ago

How climate change is affecting where species live

Human presence shifts balance between leopards and hyenas in East Africa

Physicists' laser experiment excites atom's nucleus, may enable new type of atomic clock
Relevant physicsforums posts, who chooses official designations for individual dolphins, such as fb15, f153, f286.
Jun 26, 2024
Color Recognition: What we see vs animals with a larger color range
Jun 25, 2024
Innovative ideas and technologies to help folks with disabilities
Jun 24, 2024
Is meat broth really nutritious?
Covid virus lives longer with higher co2 in the air.
Jun 22, 2024
Periodical Cicada Life Cycle
Jun 21, 2024
More from Biology and Medical
Related Stories

Investigating the ethical landscape of brain organoid research
Feb 28, 2024

Framework provides guidance for ethical wildlife management
Nov 8, 2023

Exploring ethical and legal ramifications of growing brain organoids from human fetal brain tissue
Apr 9, 2024

AI could transform ethics committees
Mar 1, 2024

Initiative encourages computer science students to incorporate ethics into their work
Apr 29, 2024

Q&A: The embedded ethics approach in AI development
Sep 1, 2020
Recommended for you

Researchers propose a new, holistic way to teach synthetic biology
Jun 27, 2024

Unlocking biodiversity insights from the tropical Andes

Biomechanics of sound production in high-pitched classical singing
Jun 18, 2024

'Sour Patch' adults: 1 in 8 grown-ups love extreme tartness, study shows

Linking environmental influences, genetic research to address concerns of genetic determinism of human behavior
Feb 27, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- Past Presentations

International Conference on Software and Systems Processes 2022
Jointly held with the international conference on global software engineering (icgse).
May 19-20, 2022 | Virtual
Call for Papers
ICSSP 2022 aims to bring together researchers and practitioners to share their research findings, experiences, and new ideas on diverse topics related to software and system processes and global software engineering. For 2022, the theme of the joint conference is: “Envisioning the Future of Software Engineering Process & Practice for Global Competitiveness and Innovation.” The goal is to advance both the state of the research and the state of the practice by applying innovative ideas from different fields of research to the future of software engineering process and globally distributed software development. Download the Call for Papers and the Industry Call for Papers .
Thank you for your interest in submitting a paper; the call for papers is now closed.
Submissions
Submissions are invited for unpublished original work in the following categories:
- Full Papers (10 pages + up to 2 pages with references) that reflect completed and evaluated research on novel approaches to major software and systems engineering process challenges, especially relating to the Future of Software Engineering Process & Practice for Global Competitiveness and Innovation. Enhanced versions of the best research papers will be included in a special issue of the Journal of Software: Evolution and Process.
- Short papers (5 pages including references) that present concise research results, describe work-in-progress (e.g. Ph. D. research), or conceptual and position papers addressing new perspectives, open questions and future directions. Short papers can also be industrial papers, for instance, describing practical challenges or research needs motivated by experience.
- Experience reports – by industry. Experience Reports provide the opportunity for you to share your practical experience through a paper and accompanying talk at the conference. An experience report is a reflection of your own industry experiences (e.g. challenges you have seen, what you tried and approaches you have taken, what worked and what didn’t work). In a specific call, we will invite submissions of an abstract in which you briefly explain your own, unpublished experience related to one or more topics of the conference. If your proposal is accepted, you will then be shepherded as you write your report. Experience reports are short papers (maximum 5 pages) that will be published in the conference proceedings. (See Industry Call for Papers .)
- Industry talk – by industry. Industry track submissions are expected to have a strong focus on the real-world application of techniques, tools, methodologies, processes, and practices. Submissions should clearly identify the problem that is addressed, the industrial context, and the outcomes, as well as the expected learning for the audience. (See Industry Call for Papers .)
- Journal first – original works published only on Journal but never presented in a conference. Specific Call To Be Announced
Topics of Interest
Submissions must be related to software and systems processes and/or global software engineering and we are especially interested in, but not limited to, papers addressing the topics in the following list:
Work from Anywhere (WFX) Emerging processes supporting remote work Infrastructure for WFX Hiring and Economics of WFX
Education and Training Software process management (SPM) education Global and Distributed SE curricula Lessons learned on SPM or GSE courses
Future and Innovation Related to Software Process and Collaboration Process for Embedded and IoT Systems ALM/PLM for distributed teams Software Processes for Augmented, virtual, and extended reality (AR/VR/ XR) Use of AR/VR/XR in Distributed Settings Software Process for low-code development practices Quantum programming and software engineering Software process for Blockchain Blockchain to improve/enhance Global Software Development Software Developer’s Experience (DX) Software Developers’ Relationship (DevRel) Process and Infrastructure for Software Applications in Scientific Research
Submission Requirements and Policies
All submissions must conform to the ICSSP (and ICGSE) 2022 formatting and submission instructions and must not exceed the given page limits for the applicable type of submission. All submissions must be in English. Page limits include all text, figures, tables, references, and appendices. Only research papers are permitted up to two more pages containing only references . The page limit is strict, and purchase of additional pages in the proceedings is not allowed at any point in the process (including after the paper is accepted). All submissions must be in PDF.
Formatting – Submissions must conform to the ACM template Formatting instructions are available at https://www.acm.org/publications/proceedings-template for both LaTeX and Word users. LaTeX users must use the provided acmart.cls and ACM-Reference-Format.bst without modification, enable the conference format in the preamble of the document (i.e., \documentclass[sigconf,review]{acmart}), and use the ACM reference format for the bibliography (i.e., \bibliographystyle{ACM-Reference-Format}). The review option adds line numbers, thereby allowing referees to refer to specific lines in their comments. In addition, a selection of the best papers from the ICSSP 2022 conference will appear in a special issue of the Journal of Software Evolution and Process.
Authorship policy – By submitting to ICSSP (and ICGSE) 2022, authors acknowledge that they conform to the authorship policy of the ACM . Note in particular that submitted papers must reflect the original work of the authors, the authors must be entitled to publish the work, the work must not have been published previously in a refereed or formally reviewed publication, and the work must not be in press elsewhere while under review for ICSSP 2022, among other provisions.
Plagiarism policy – By submitting to ICSSP 2022, authors also acknowledge that they are aware of and agree to be bound by the ACM Policy and Procedures on Plagiarism . In particular, papers submitted to ICSSP and ICGSE 2022 must not have been published elsewhere and must not be under review or submitted for review elsewhere whilst under consideration for ICSSP or ICGSE 2022. Contravention of this concurrent submission policy will be deemed a serious breach of scientific ethics, and appropriate action will be taken in all such cases. To check for double submission and plagiarism issues, the chairs reserve the right to (1) share the list of submissions with the PC Chairs of other conferences with overlapping review periods and (2) use external plagiarism detection software, under contract to the ACM, to detect violations of these policies.
Submission site – Submissions must be made through the ICSSP (and ICGSE) 2022 submission site on EasyChair no later than the applicable submission deadline. Be sure to direct your submissions correctly and select the appropriate submission type when uploading your submission. We encourage authors to upload their paper info early (and can submit the PDF later, but still before or on the date of the submission deadline AoE) to support the management of the review process.
Any submission that does not comply with these requirements may be desk rejected by the PC Co-Chairs without further review.
Notice: The official publication date is the first day of the conference. The official publication date affects the deadline for any patent filings related to published work.
Proceedings – The proceedings will be published by ACM, and will appear in the ACM digital library. The proceedings will only contain papers that have been presented at ICSSP 2022 and for which at least one author has registered.
Reviewing – All submissions will be peer-reviewed by the ICSSP or ICGSE 2022 program committee members. When submitting, you will have the option to choose if you are submitting to ICSSP or to ICGSE as a track. Each submitted research paper will receive reviews from at least three members of the specific program committee.
ICSSP 2022 does not employ double-blind review process .
Requirement to Register, Attend, and Present – Upon notification of acceptance, authors of accepted papers will be asked to complete a copyright form and will receive further instructions for preparing their camera-ready versions. At least one author of each accepted paper is expected to register for and attend the ICSSP 2022 and present the work. The organizers have the right to pull papers out of the proceedings if there is no associated registration by two weeks past the authors’ registration deadline. All accepted contributions with at least one registered author will be published in the conference electronic proceedings and appear in the ACM digital library.
Open Science Policy – ICSSP (and ICGSE) 2022 encourage authors to submit artifact packages and/or data sets with their papers, for the sake of transparency, reusability, replicability, and/or reproducibility, as well as for facilitating the peer review process. The following guidelines are recommendations and are not mandatory. Your choice will not affect the review process for your paper. Should you decide to embrace this policy, we strongly encourage you to archive empirical datasets on Zenodo or Dataverse , following Google guidelines to dataset providers , and share analysis scripts on freely accessible code repositories such as GitHub, GitLab, and Bitbucket.
Self-Archiving – ICSSP and ICGSE 2022 also encourage authors to self-archive a preprint of your accepted manuscript in arXiv.org or other similar open repositories. This is permitted by the ACM publisher. Note that the final version of the paper, as laid out by the publisher, cannot be self-archived. Instead, manuscripts with reviewer comments addressed must be used, but before applying the camera-ready instructions and templates.
Contact – Feel free to contact the ICSSP and ICGSE 2022 Program Co-Chairs for more details.
Reaction Mechanism of Actin ATP Hydrolysis Studied by QM/MM Calculations
- Published: 02 July 2024
Cite this article

- Yiwen Wang 1 ,
- Lirui Lin 2 , 3 ,
- Li-Yan Xu 4 , 5 , 6 ,
- En-Min Li 1 , 4 &
- Geng Dong 1 , 3 , 6
Actin fibers are an important part of the cytoskeleton, providing vital support for the plasma membrane. This function is driven by its ATPase (ATP: adenosine triphosphate) activity, i.e. , ATP+H 2 O→ADP+P i . This seemingly simple reaction has attracted much attention because the hydrolysis of ATP provides energy to support life processes. However, the reaction mechanism of ATP hydrolysis in actin is not clear. In order to gain deep insights into the functions of actin, it is essential to elucidate the reaction mechanism of the actin ATP hydrolysis. In this paper, we have studied the reaction mechanism of the ATP hydrolysis in actin by the combined quantum mechanical and molecular mechanics (QM/MM) calculations. Our results show that 1) bond cleavage of the P γ —O S of ATP and bond formation between oxygen of the lytic water and P γ atoms take place simultaneously, and this is the rate-limiting step of the hydrolysis; 2) the proton on the lytic water transfers to the phosphate to form H 2 P γ O 4 − via one bridge water. The energy barrier of the complete reaction is 17.6 kcal/mol (1 kcal=4.184 kJ), which is in high agreement with the experimental value.

This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or Ebook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Blanchoin L., Pollard T. D., Biochemistry , 2002 , 41 , 597.
Article CAS PubMed Google Scholar
Vorobiev S., Strokopytov B., Drubin D. G., Frieden C., Ono S., Condeelis J., Rubenstein P. A., Almo S. C., Proc. Natl. Acad. Sci. , 2003 , 100 , 5760.
Article CAS PubMed PubMed Central Google Scholar
Akola J., Jones R. O., J. Phys. Chem. B , 2006 , 110 , 8121.
Oda T., Iwasa M., Aihara T., Maéda Y., Narita A., Nature , 2009 , 457 , 441.
Pfaendtner J., Lyman E., Pollard T. D., Voth G. A., J. Mol. Biol. , 2010 , 396 , 252.
Freedman H., Laino T., Curioni A., J. Chem. Theory Comput. , 2012 , 8 , 3373.
McCullagh M., Saunders M. G., Voth G. A., J. Am. Chem. Soc. , 2014 , 136 , 13053.
Kanematsua Y., Narita A., Odae T., Koike R., Ota M., Takano Y., Moritsugu K., Fujiwara I., Tanaka K., Komatsu H., Nagae T., Watanabe N., Iwasa M., Maeda Y., Takeda S., Proc. Natl. Acad. Sci. , 2022 , 119 , 40.
Google Scholar
Ogrizek M., Janežič M., Valjavec K., Perdih A., J. Chem. Inf. Model. , 2022 , 62 , 3896.
Grigorenko B. L., Rogov A. V., Topol I. A., Burt S. K., Martinez H. M., Nemukhin A. V., Proc. Natl. Acad. Sci. , 2007 , 104 , 7057.
Manna R. N., Dutta M., Jana B., Phys. Chem. Chem. Phys. , 2020 , 22 , 1534.
Parke C. L., Wojcik E. J., Kim S., Worthylake D. K., J. Biol. Chem. , 2010 , 285 , 5859.
Hayashi S., Ueno H., Shaikh A. R., Umemura M., Kamiya M., Ito Y., Ikeguchi M., Komoriya Y., Iino R., Noji H., J. Am. Chem. Soc. , 2012 , 134 , 8447.
Oosterheert W., Klink B. U., Belyy A., Pospich S., Raunser S., Nature , 2022 , 611 , 374.
Yang Z., Lasker K., Schneidman-Duhovny D., Webb B., Huang C. C., Pettersen E. F., Goddard T. D., Meng E. C., Sali A., Ferrin T. E., J. Struct. Biol. , 2012 , 179 , 269.
Li H., Robertson A. D., Jensen J. H., Proteins , 2005 , 61 , 704.
Salomon-Ferrer R., Götz A. W., Poole D., Le Grand S., Walker R. C., J. Chem. Theory Comput. , 2013 , 9 , 3878.
Maier J. A., Martinez C., Kasavajhala K., Wickstrom L., Hauser K., Simmerling C., J. Chem. Theory Comput. , 2015 , 11 , 3696.
Jorgensen W. L., Chandrasekhar J., Madura J. D., Impey R. W., Klein M. L., J. Chem. Phys. , 1983 , 79 , 926.
Article CAS Google Scholar
Götz A. W., Williamson M. J., Xu D., Poole D., Le Grand S., Walker R. C., J. Chem. Theory Comput. , 2012 , 8 , 1542.
Article PubMed PubMed Central Google Scholar
Le Grand S., Götz A. W., Walker R. C., Comput. Phys. Commun. , 2013 , 184 , 374.
Darden T., York D., Pedersen L., J. Chem. Phys. , 1993 , 98 , 10089.
Lambrakos S. G., Boris J. P., Oran E. S., Chandrasekhar I., Nagumo M., J. Comput. Phys. , 1989 , 85 , 473.
Article Google Scholar
Izaguirre J. A., Catarello D. P., Wozniak J. M., Skeel R. D., J. Chem. Phys. , 2001 , 114 , 2090.
Perdew J. P., Burke K., Ernzerhof M., Phys. Rev. Lett. , 1996 , 77 , 3865.
Schäfer A., Horn H., Ahlrichs R., J. Chem. Phys. , 1992 , 97 , 2571.
Becke A. D., Phys. Rev. A , 1988 , 38 , 3098.
Lee C., Yang W. T., Parr R. G., Phys. Rev. B , 1988 , 37 , 785.
Becke A. D., J. Chem. Phys. , 1993 , 98 , 5648.
Weigend F., Ahlrichs R., Phys. Chem. Chem. Phys. , 2005 , 7 , 3297.
Neese F., Wiley Interdiscip. Rev. Comput. Mol. Sci. , 2022 , 12 , e1606.
Neese F., Wiley Interdiscip. Rev. Comput. Mol. Sci. , 2017 , 8 , e1327.
Sun R., Sode O., Dama J. F., Voth G. A., J. Chem. Theory Comput. , 2017 , 13 , 2332.
Harrison C. B., Schulten K., J. Chem. Theory Comput. , 2012 , 8 , 2328.
Prasad B. R., Plotnikov N. V., Warshel A., J. Phys. Chem. B , 2013 , 117 , 153.
Wang C., Huang W. T., Liao J. L., J. Phys. Chem. B , 2015 , 119 , 3720.
McGrath M. J., Kuo I. F., Hayashi S., Takada S., J. Am. Chem. Soc. , 2013 , 135 , 8908.
Admiraal S. J., Herschlag D., Chem. Biol. , 1995 , 2 , 729.
Download references
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21907063), the Li Ka-shing Foundation, China (No. LD0101), the 2020 Li Ka-shing Foundation Cross-Disciplinary Research Grant, China (No. 2020LKSFG07B), the Innovation Team Grant of Department of Education of Guangdong Provice, China (No. 2021KCXTD005) and the Shantou University Medical College (SUMC) Scientific Research Initiation Grant, China (No. 510858063).
We acknowledge the Network and Information Center for providing the resources on computations and storage.
Author information
Authors and affiliations.
Department of Biochemistry and Molecular Biology, Shantou University Medical College, Shantou, 515041, P. R. China
Yiwen Wang, En-Min Li & Geng Dong
Department of Bioinformatics, Shantou University Medical College, Shantou, 515041, P. R. China
Medical Informatics Research Center, Shantou University Medical College, Shantou, 515041, P. R. China
Lirui Lin & Geng Dong
Key Laboratory of Molecular Biology in High Cancer Incidence Coastal Area of Guangdong Higher Education Institutes, Shantou University Medical College, Shantou, 515041, P. R. China
Li-Yan Xu & En-Min Li
Cancer Research Center, Shantou University Medical College, Shantou, 515041, P. R. China
Guangdong Provincial Key Laboratory of Infectious Diseases and Molecular Immunopathology, Shantou University Medical College, Shantou, 515041, P. R. China
Li-Yan Xu & Geng Dong
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Lirui Lin , En-Min Li or Geng Dong .
Ethics declarations
The authors declare no conflicts of interest.
Supplementary Information
Reaction mechanism of actin atp hydrolysis studied by qm/mm calculations, rights and permissions.
Reprints and permissions
About this article
Wang, Y., Lin, L., Xu, LY. et al. Reaction Mechanism of Actin ATP Hydrolysis Studied by QM/MM Calculations. Chem. Res. Chin. Univ. (2024). https://doi.org/10.1007/s40242-024-4089-2
Download citation
Received : 10 April 2024
Accepted : 06 June 2024
Published : 02 July 2024
DOI : https://doi.org/10.1007/s40242-024-4089-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Adenosine triphosphate (ATP) hydrolysis
- Quantum mechanical and molecular mechanics (QM/MM)
- Reaction mechanism
- Find a journal
- Publish with us
- Track your research
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Research ethics.
Jennifer M. Barrow ; Grace D. Brannan ; Paras B. Khandhar .
Affiliations
Last Update: September 18, 2022 .
- Introduction
Multiple examples of unethical research studies conducted in the past throughout the world have cast a significant historical shadow on research involving human subjects. Examples include the Tuskegee Syphilis Study from 1932 to 1972, Nazi medical experimentation in the 1930s and 1940s, and research conducted at the Willowbrook State School in the 1950s and 1960s. [1] As the aftermath of these practices, wherein uninformed and unaware patients were exposed to disease or subject to other unproven treatments, became known, the need for rules governing the design and implementation of human-subject research protocols became very evident.
The first such ethical code for research was the Nuremberg Code, arising in the aftermath of Nazi research atrocities brought to light in the post-World War II Nuremberg Trials. [1] This set of international research standards sought to prevent gross research misconduct and abuse of vulnerable and unwitting research subjects by establishing specific human subject protective factors. A direct descendant of this code was drafted in 1978 in the United States, known as the Belmont Report, and this legislation forms the backbone of regulation of clinical research in the USA since its adoption. [2] The Belmont Report contains 3 basic ethical principles:
- Respect for persons
- Beneficence
Additionally, the Belmont Report details research-based protective applications for informed consent, risk/benefit assessment, and participant selection. [3]
- Issues of Concern
The first protective principle stemming from the 1978 Belmont Report is the principle of Respect for Persons, also known as human dignity. [2] This dictates researchers must work to protect research participants' autonomy while also ensuring full disclosure of factors surrounding the study, including potential harms and benefits. According to the Belmont Report, "an autonomous person is an individual capable of deliberation about personal goals and acting under the direction of such deliberation." [1]
To ensure participants have the autonomous right to self-determination, researchers must ensure that potential participants understand that they have the right to decide whether or not to participate in research studies voluntarily and that declining to participate in any research does not affect in any way their access to current or subsequent care. Also, self-determined participants must be able to ask the researcher questions and comprehend the questions asked by the researcher. Researchers must also inform participants that they may stop participating in the study without fear of penalty. [4] As noted in the Belmont Report definition above, not all individuals can be autonomous concerning research participation. Whether because of the individual's developmental level or because of various illnesses or disabilities, some individuals require special research protections that may involve exclusion from research activities that can cause potential harm or appointing a third-party guardian to oversee the participation of such vulnerable persons. [5]
Researchers must also ensure they do not coerce potential participants into agreeing to participate in studies. Coercion refers to threats of penalty, whether implied or explicit, if participants decline to participate or opt out of a study. Additionally, giving potential participants extreme rewards for agreeing to participate can be a form of coercion. The rewards may provide an enticing enough incentive that the participant feels they need to participate. In contrast, they would otherwise have declined if such a reward were not offered. While researchers often use various rewards and incentives in studies, they must carefully review this possibility of coercion. Some incentives may pressure potential participants into joining a study, thereby stripping participants of complete self-determination. [3]
An additional aspect of respecting potential participants' self-determination is to ensure that researchers have fully disclosed information about the study and explained the voluntary nature of participation (including the right to refuse without repercussion) and possible benefits and risks related to study participation. A potential participant cannot make a truly informed decision without complete information. This aspect of the Belmont Report can be troublesome for some researchers based on their study designs and research questions. Noted biases related to reactivity may occur when study participants know the exact guiding research questions and purposes. Some researchers may avoid reactivity biases using covert data collection methods or masking critical study information. Masking frequently occurs in pharmaceutical trials with placebos because knowledge of placebo receipt can affect study outcomes. However, masking and concealed data collection methods may not fully respect participants' rights to autonomy and the associated informed consent process. Any researcher considering hidden data collection or masking of some research information from participants must present their plans to an Institutional Review Board (IRB) for oversight, as well as explain the potential masking to prospective patients in the consent process (ie, explaining to potential participants in a medication trial that they are randomly assigned either the medication or a placebo). The IRB determines if studies warrant concealed data collection or masking methods in light of the research design, methods, and study-specific protections. [6]
The second Belmont Report principle is the principle of beneficence. Beneficence refers to acting in such a way to benefit others while promoting their welfare and safety. [7] Although not explicitly mentioned by name, the biomedical ethical principle of nonmaleficence (not harm) also appears within the Belmont Report's section on beneficence. The beneficence principle includes 2 specific research aspects:
- Participants' right to freedom from harm and discomfort
- Participants' rights to protection from exploitation [8]
Before seeking IRB approval and conducting a study, researchers must analyze potential risks and benefits to research participants. Examples of possible participant risks include physical harm, loss of privacy, unforeseen side effects, emotional distress or embarrassment, monetary costs, physical discomfort, and loss of time. Possible benefits include access to a potentially valuable intervention, increased understanding of a medical condition, and satisfaction with helping others with similar issues. [8] These potential risks and benefits should explicitly appear in the written informed consent document used in the study. Researchers must implement specific protections to minimize discomfort and harm to align with the principle of beneficence. Under the principle of beneficence, researchers must also protect participants from exploitation. Any information provided by participants through their study involvement must be protected.
The final principle contained in the Belmont Report is the principle of justice, which pertains to participants' right to fair treatment and right to privacy. The selection of the types of participants desired for a research study should be guided by research questions and requirements not to exclude any group and to be as representative of the overall target population as possible. Researchers and IRBs must scrutinize the selection of research participants to determine whether researchers are systematically selecting some groups (eg, participants receiving public financial assistance, specific ethnic and racial minorities, or institutionalized) because of their vulnerability or ease of access. The right to fair treatment also relates to researchers treating those who refuse to participate in a study fairly without prejudice. [3]
The right to privacy also falls under the Belmont Report's principle of justice. Researchers must keep any shared information in their strictest confidence. Upholding the right to privacy often involves procedures for anonymity or confidentiality. For participants' data to be completely anonymous, the researcher cannot have the ability to connect the participants to their data. The study is no longer anonymous if researchers can make participant-data connections, even if they use codes or pseudonyms instead of personal identifiers. Instead, researchers are providing participant confidentiality. Various methods can help researchers assure confidentiality, including locking any participant identifying data and substituting code numbers instead of names, with a correlation key available only to a safety or oversight functionary in an emergency but not readily available to researchers. [3]
- Clinical Significance
One of the most common safeguards for the ethical conduct of research involves using external reviewers, such as an Institutional Review Board (IRB). Researchers seeking to begin a study must submit a full research proposal to the IRB, which includes specific data collection instruments, research advertisements, and informed consent documentation. The IRB may perform a complete or expedited review depending on the nature of the study and the risks involved. Researchers cannot contact potential participants or start collecting data until they obtain full IRB approval. Sometimes, multi-site studies require approvals from several IRBs, which may have different forms and review processes. [3]
A significant study aspect of interest to IRB members is using participants from vulnerable groups. Vulnerable groups may include individuals who cannot give fully informed consent or those individuals who may be at elevated risk of unplanned side effects. Examples of vulnerable participants include pregnant women, children younger than the age of consent, terminally ill individuals, institutionalized individuals, and those with mental or emotional disabilities. In the case of minors, assent is also an element that must be addressed per Subpart D of the Code of Federal Regulations, 45 CFR 46.402, which defines consent as "a child's affirmative agreement to participate in research; mere failure to object should not, absent affirmative agreement, be construed as assent." [9] There is a lack in the literature on when minors can understand research, although current research suggests that the age by which a minor could assent is around 14. [10] Anytime researchers include vulnerable groups in their studies, they must have extra safeguards to uphold the Belmont Report's ethical principles, especially beneficence. [3]
- Enhancing Healthcare Team Outcomes
Research ethics is a foundational principle of modern medical research across all disciplines. The overarching body, the IRB, is intentionally comprised of experts across various disciplines, including ethicists, social workers, physicians, nurses, other scientific researchers, counselors, mental health professionals, and advocates for vulnerable subjects. There is also often a legal expert on the panel or available to discuss any questions regarding the legality or ramifications of studies.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Jennifer Barrow declares no relevant financial relationships with ineligible companies.
Disclosure: Grace Brannan declares no relevant financial relationships with ineligible companies.
Disclosure: Paras Khandhar declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Barrow JM, Brannan GD, Khandhar PB. Research Ethics. [Updated 2022 Sep 18]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- The historical, ethical, and legal background of human-subjects research. [Respir Care. 2008] The historical, ethical, and legal background of human-subjects research. Rice TW. Respir Care. 2008 Oct; 53(10):1325-9.
- The Belmont Report at 40: Reckoning With Time. [Am J Public Health. 2018] The Belmont Report at 40: Reckoning With Time. Adashi EY, Walters LB, Menikoff JA. Am J Public Health. 2018 Oct; 108(10):1345-1348. Epub 2018 Aug 23.
- Informed consent in human experimentation before the Nuremberg code. [BMJ. 1996] Informed consent in human experimentation before the Nuremberg code. Vollmann J, Winau R. BMJ. 1996 Dec 7; 313(7070):1445-9.
- Review The History of Human Subjects Research and Rationale for Institutional Review Board Oversight. [Nutr Clin Pract. 2021] Review The History of Human Subjects Research and Rationale for Institutional Review Board Oversight. Spellecy R, Busse K. Nutr Clin Pract. 2021 Jun; 36(3):560-567. Epub 2021 Jan 13.
- Review Ethical issues in research. [Best Pract Res Clin Obstet Gyn...] Review Ethical issues in research. Artal R, Rubenfeld S. Best Pract Res Clin Obstet Gynaecol. 2017 Aug; 43:107-114. Epub 2017 Jan 23.
Recent Activity
- Research Ethics - StatPearls Research Ethics - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- JME Commentaries
- BMJ Journals
You are here
- Online First
- Reviewing past and present consent practices in unplanned obstetric interventions: an eye towards the future
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0002-2780-7897 Morganne Wilbourne 1 ,
- http://orcid.org/0000-0002-9347-8702 Frances Hand 2 ,
- Sophie McAllister 3 ,
- Louise Print-Lyons 4 ,
- Meena Bhatia 3
- 1 Women's and Reproductive Health , Oxford University , Oxford , UK
- 2 Faculty of Law , University of Oxford , Oxford , UK
- 3 Obstetrics and Gynaecology , Oxford University Hospitals NHS Foundation Trust , Oxford , UK
- 4 Oxfordshire Maternity and Neonatal Voices Partnership , Oxford , UK
- Correspondence to Morganne Wilbourne, Women's and Reproductive Health, Oxford University, Oxford OX3 9DU, UK; morganne.wilbourne{at}spc.ox.ac.uk
Many first-time mothers (primiparous) within UK National Health Service (NHS) settings require an obstetric intervention to deliver their babies safely. While the antepartum period allows time for conversations about consent for planned interventions, such as elective caesarean section, current practice is that, in emergencies, consent is addressed in the moments before the intervention takes place. This paper explores whether there are limitations on the validity of consent offered in time-pressured and emotionally charged circumstances, specifically concerning emergency obstetric interventions. Using the legal framework of the Mental Capacity Act, Montgomery v. Lanarkshire Health Board (2015) and McCulloch v Forth Valley Health Board (2023), we argue that while women have the capacity to consent during labour, their autonomy is best supported by providing more information about instrumental delivery (ID) during the antepartum period. This conclusion is supported by some national guidelines, including those developed by the Royal College of Obstetricians and Gynaecologists, but not all. Further, we examine the extent to which these principles are upheld in modern-day practice. Data suggest there is relatively little antepartum information provision regarding ID within NHS settings, and that primiparous women do not report a thorough understanding of ID before labour. Based on these results, and bearing in mind the pressures under which NHS obstetric services currently operate, we recommend further research into patient and clinician perceptions of the consent process for ID. Pending these results, we discuss possible modes of information delivery in future practice.
- Decision Making
- Ethics- Medical
Data availability statement
No data are available.
https://doi.org/10.1136/jme-2024-109997
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
X @FrancesHand_
Contributors FH and MW have contributed equally to this project and should be considered to have joint first authorship. They are entitled to reference their own name first on curricula vitae. They have planned the project and drafted the original manuscript. SA, LP-L and MB provided revisions to the original manuscript. LP-L provided data from the Oxfordshire Maternity and Neonatal Voices Partnership. MB and SM provided supervision. MB and SM provided clinical insight and ensured accuracy with respect to current NHS practice in obstetrics. MB is the guarantor and accepts full responsibility for the finished work and the conduct of the study, had access to the data and controlled the decision to publish.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:

IMAGES
VIDEO
COMMENTS
Introduction. Ethics are a guiding principle that shapes the conduct of researchers. It influences both the process of discovery and the implications and applications of scientific findings 1.Ethical considerations in research include, but are not limited to, the management of data, the responsible use of resources, respect for human rights, the treatment of human and animal subjects, social ...
Abstract. Ethics, as an integral component of human decision-making, undeniably shape the landscape of scientific research. This article delves deeply into the nuanced realm of ethical ...
This paper describes the basic principles of Western research ethics - respect for persons, beneficence, and justice - and how the principles may be contextualized in different settings, by researchers of various backgrounds with different funding streams. Examples of lapses in ethical practice of research are used to highlight best practices.
Research Ethics is aimed at all readers and authors interested in ethical issues in the conduct of research, the regulation of research, the procedures and process of ethical review as well as broader ethical issues related to research such as scientific … | View full journal description. This journal is a member of the Committee on ...
According to Resnik (2011), many people think of ethics as a set of rules distinguishing right from wrong, but actually the term "ethics" refers to norms of conduct or of action and in disciplines of study. Research ethics or norms promote the "knowledge, truth, and avoidance of error" (p. 1) and protect against "fabricating ...
Revised on May 9, 2024. Ethical considerations in research are a set of principles that guide your research designs and practices. Scientists and researchers must always adhere to a certain code of conduct when collecting data from people. The goals of human research often include understanding real-life phenomena, studying effective treatments ...
Introduction. Research includes a set of activities in which researchers use various structured methods to contribute to the development of knowledge, whether this knowledge is theoretical, fundamental, or applied (Drolet & Ruest, accepted).University research is carried out in a highly competitive environment that is characterized by ever-increasing demands (i.e., on time, productivity ...
Abstract. The Oxford Handbook of Research Ethics provides a critical overview of the ethics of human subjects research within multiple disciplines and fields, including biomedicine, public health, psychiatry, sociology, political science, and public policy. Featuring forty-four innovative chapters by leading research ethicists, it aims to improve scholarship in research ethics by encouraging ...
Cross-border data sharing through the lens of research ethics committee members in sub-Saharan Africa. Nezerith Cengiz, Siti M. Kabanda, Keymanthri Moodley. The role of pain self-efficacy and pain catastrophising in the relationship between chronic pain and depression: A moderated mediation model.
Research ethics in international and comparative education (ICE) highlights the diverse challenges that ICE researchers face in enacting ethical practice. In particular, the significant gaps between ethics presented in Western ethical guidelines and international fieldwork. Through analysis of existing guidelines and questionnaire responses ...
The search was made on September 22, 2020 using the descriptors "research ethics ... The reflection on the researcher's role and his/her influence on the research field was the theme of the paper by Råheim et al. (2016). During two years, a group of six experienced researchers had meetings to debate this theme based on studies with ...
100 Questions (and Answers) About Research Ethics is an essential guide for graduate students and researchers in the social and behavioral sciences. It identifies ethical issues that individuals must consider when planning research studies as well as provides guidance on how to address ethical issues that might arise during research implementation.
Ethics of Science is a comprehensive and student-friendly introduction to the study of ethics in science and scientific research. The book covers: * Science and Ethics * Ethical Theory and ...
15. Ethical practice in scientific research should be carried out as. an " ethical process " in matters related to authorization and. informed consent of those involved (Kim, et al. 2009 ...
Individual responsibilities to enhance RRP. As explained in Fig. 1, a successfully governed research should consider ethics at the planning stages prior to research.Many international guidance are compatible in enforcing/recommending 14 different "responsibilities" that were first highlighted in the Singapore Statement (2010) for researchers to follow and achieve competency in RRP.
More broadly, there was a fourfold increase in retractions of biomedical research papers in Europe between 2000 and 2021, most of which related to research misconduct (Nature 630, 280-281; 2024 ...
Five Dimensions of Research Ethics. We have identified five core dimensions of research ethics: (1) normative ethics, which includes meta-ethical questions; (2) compliance with regulations, statutes, and institutional policies; (3) the rigor and reproducibility of science; (4) social value; and (5) workplace relationships.
She has just published a paper on the relationship between pesticide exposure and PD in a prestigious journal. She is planning to publish many other papers from her dataset. She receives a request from another research team that wants access to her complete dataset. ... Education in research ethics is can help people get a better understanding ...
Research ethics are a set of principles and guidelines that shape and guide the way any research involving sentient beings (i.e. people and animals) is designed, conducted, managed, used and disseminated. In these guidelines, the term 'research' is used broadly: it includes diagnostic and explorative investigations of social issues of ...
Ethics in research and how it is translated into practice is fundamental to rule out any potential misconduct either with the scientific method or the way results are presented to the world, thus impacting patients outcomes. ... Supposedly high-profile papers with the antimalarial medication hydroxychloroquine either favoring its use or ...
Ethics or moral philosophy is a branch of philos-. ophy with standards or codes or value systems. and involves defending, systematizing, recommending concepts of right, and minimizing. wrong ...
Ethical Considerations. Ethical considerations in research refer to the principles and guidelines that researchers must follow to ensure that their studies are conducted in an ethical and responsible manner. These considerations are designed to protect the rights, safety, and well-being of research participants, as well as the integrity and credibility of the research itself
To ensure this is most functional, "The Collaborative Ethics effort is also aligned with the Wyss Institute's Technology Innovation Funnel, which formalizes the journey of its cutting-edge ...
Abstract. Published articles in scientific journals are a key method for knowledge-sharing. Researchers can face the pressures to publish and this can sometimes lead to a breach of ethical values, whether consciously or unconsciously. The prevention of such practices is achieved by the application of strict ethical guidelines applicable to ...
Full and Short Research Papers Abstract Submission: January 24, 2022 (optional) Paper Submission: January 24, 2022 Notification: March 4, 2022 ... Accountability, Transparency, and Ethics in Software Process and Development Engineering societal-scale software systems Fairness in software process Processes to assure Equity in Software Development
See our research paper for more details. Limitations. We trained CriticGPT on ChatGPT answers that are quite short. To supervise the agents of the future, we will need to develop methods that can help trainers to understand long and complex tasks. ... In our research on CriticGPT, we found that applying RLHF to GPT-4 has promise to help humans ...
This work was supported by the National Natural Science Foundation of China (No. 21907063), the Li Ka-shing Foundation, China (No. LD0101), the 2020 Li Ka-shing Foundation Cross-Disciplinary Research Grant, China (No. 2020LKSFG07B), the Innovation Team Grant of Department of Education of Guangdong Provice, China (No. 2021KCXTD005) and the Shantou University Medical College (SUMC) Scientific ...
Research ethics is a foundational principle of modern medical research across all disciplines. The overarching body, the IRB, is intentionally comprised of experts across various disciplines, including ethicists, social workers, physicians, nurses, other scientific researchers, counselors, mental health professionals, and advocates for ...
Many first-time mothers (primiparous) within UK National Health Service (NHS) settings require an obstetric intervention to deliver their babies safely. While the antepartum period allows time for conversations about consent for planned interventions, such as elective caesarean section, current practice is that, in emergencies, consent is addressed in the moments before the intervention takes ...