Writing A Book Title In Your Essay – The Right Way
Table of contents
- 1 APA Style: How to Write Book Titles in Essays
- 2 APA Style Essay: Writing The Name of The Author
- 3 MLA Style Essay: Citing a Book Title
- 4 Chicago Style Essay: Writing the Book Title
- 5 Writing Various Types of Titles
- 6 Should We Underline or Italicize Book Titles?
When you are writing an academic essay , the book title and author’s name should be written in italics. However, if the book title is part of a larger work (such as a journal article), it should be underlined instead. So, you’re wondering how to write a book title in an essay?
Writing an essay with a book title can be tricky, particularly because each style guide has its own formatting rules for including titles in the main text. Whether you are using MLA, APA, Chicago, or Harvard referencing styles, you will need to consider how to properly format the book title. For more complicated literature-based assignments, seeking assistance from an admission essay writing service may be wise, as they specialize in writing essays that incorporate academic sources.
In this article, we will explore how to write both titles in an essay properly so that you avoid any mistakes!

APA Style: How to Write Book Titles in Essays
When writing an essay, you must follow the style guide provided by your professor. Some teachers may require you to use APA style and others MLA style. There are some rules on how to quote a book title in an essay. You should use italics and quotation marks when writing book titles in essays. For example: “ The Rape of Nanking: The Forgotten Holocaust of World War II. “
When writing a book title in APA Style , you should be aware of these rules:
Write the book title in italics and place it after the author’s name, which is presented in reverse order (last name first).
Use quotation marks around the headline of a chapter or article.
Capitalize proper names that are not common nouns (names of people, places, organizations), but do not capitalize words such as “and,” “or,” “to,” or “and/or.”
Do not capitalize prepositions that appear at the beginning of titles if they are followed by an article (e.g., “A,” “An”), but do capitalize prepositions at the beginning of titles if they are not followed by articles (“Of”).
The first word of the headline should be capitalized, as well as any other words after a colon or hyphen. For example, “The Elements of Style: Grammar for Everyone” or “Theories of Personality: Critical Perspectives.”
Capitalize proper names and words derived from them (e.g., the names of people, places, organizations), except proper nouns used generically (e.g., ‘a bed’).
APA Style Essay: Writing The Name of The Author
You should always use the full name and surname of the author in your APA essay because this will give proper credit to the writer. If you do not mention the author’s full name, people may not know who wrote what and will think you copied it from somewhere else. This will cause lots of problems for you and your reputation as well.
Make sure that all authors’ names appear in the same format in each entry. For example, if one person’s surname is Smith and another’s is Jones, both have first names starting with “J.” It may seem like they are being cited as different people when they’re actually written differently from each other on separate pages in your paper.
To write an APA essay without any issues, there are certain rules that you need to follow while writing an author’s name in APA essay:
- Use only one author’s name in your paper unless there are multiple authors
- If there are multiple authors, then use both their last names followed by the initials of their first names
- Only use initials of first names when there are three or more authors; otherwise, use full names with their last names
Example: Johnson, M.C., Carlson, M., Smith, J. N., & Hanover, L. E.
MLA Style Essay: Citing a Book Title
Now let’s discuss how to mention a book in an essay. The MLA Handbook for Writers of Research Papers, 7th edition, published by the Modern Language Association (2014), contains detailed rules about how to cite a book title in an essay.
The following guidelines will instruct you on how to refer to a book in an essay in MLA style :
- List your sources at the end of your paper, before the works cited page or bibliography.
- Use italics for titles of books, magazines, and newspapers, but not for articles within those publications, which should be placed in quotation marks.
- Include all relevant book information under two categories: “title” and “author.” In the former category, include the work’s title and its subtitle if there is one; do this even if neither appears on your title page (see below). In the latter category, include only primary authors who have written or edited an entire book; if there are multiple contributors, you should cite them separately under each.
The general format for citing the title of the book in an essay is as follows:
Author’s last name, first initial (Date). Title of Book with Subtitle if there is one. Publisher Name/Location of Publisher; Year Published
Chicago Style Essay: Writing the Book Title
One of the most important things to remember when writing in Chicago style is how to write the title of a book in an essay. To write a good book title in an essay, you should follow these steps:
- Write it at the beginning of your sentence.
- Capitalize it just like any other noun or proper noun.
- Put a comma after the title unless it’s an introductory clause or phrase. For example: “The Firm,” by John Grisham (not “by”) and “The Catcher in the Rye,” by J.D Salinger (not “and”).
- In addition to the book’s name, punctuation marks should also be italicized.
For example: Harry Potter and the Half-blood Prince: Children’s Edition
Writing Various Types of Titles
Now that we covered how to write a book title and author in an essay, it’s time to look at some different types of titles. When you write a book title in an essay, several things must be considered. Whether it’s a book, series, chapter title, editor’s name, or author’s name, how you write it depends on where it appears in your paper.
Here are some key rules for writing headings for novels:
- Use capital letters to write the title of the novel. For example, The Secret Garden by Frances Hodgson Burnett .
- Use italics and capital letters to write the name of the author and his/her other works mentioned in a book title—for example, Jane Austen’s Pride and Prejudice (1813) .
You should use quotation marks when writing headings of short title poems, articles, and stories.
However, before deciding which format to use, it is important to understand the main idea you want to express in your essay. Additionally, you could use essay papers for sale to help you accomplish your goal of writing an essay effectively.

Should We Underline or Italicize Book Titles?
It depends on which style guide you use. The Modern Language Association and Chicago Manual of Style both suggest using italics, while the American Psychological Association suggests using quotation marks with a few exceptions.
The way you write the title of a book in an essay is different depending on the instructions you were given. For example, if you’re writing an essay in APA style, use quotation marks around the book’s name. If you’re writing for MLA or Chicago style , however, italicize the book’s name instead. If you’re writing a handwritten essay instead of using a computer, capitalize and underline the book’s name.
Readers also enjoyed

WHY WAIT? PLACE AN ORDER RIGHT NOW!
Just fill out the form, press the button, and have no worries!
We use cookies to give you the best experience possible. By continuing we’ll assume you board with our cookie policy.
- Link to facebook
- Link to linkedin
- Link to twitter
- Link to youtube
- Writing Tips
How to Write Book Titles in Your Essays

3-minute read
- 26th May 2023
When writing an essay, you’re likely to mention other authors’ works, such as books, papers, and articles. Formatting the titles of these works usually involves using quotation marks or italics.
So how do you write a book title in an essay? Most style guides have a standard for this – be sure to check that first. If you’re unsure, though, check out our guide below.
Italics or Quotation Marks?
As a general rule, you should set titles of longer works in italics , and titles of shorter works go in quotation marks . Longer works include books, journals, TV shows, albums, plays, etc. Here’s an example of a book mention:
Shorter works include poems, articles, chapters of books, episodes of TV shows, songs, etc. If it’s a piece that’s part of a biggHow to Write Book Titles in Your Essayser work, the piece considered a short work:
Exceptions to the Rule
The rule for writing book titles in italics applies specifically to running text . If the book title is standing on its own, as in a heading, there’s no need to italicize it.
Additionally, if the book is part of a larger series and you’re mentioning both the title of the series and that of the individual book, you can consider the book a shorter work. You would set the title of the series in italics and place the book title in quotation marks:
Punctuation in Book Titles
Do you need to apply italics to the punctuation in a book title? The short answer is yes – but only if the punctuation is part of the title:
If the punctuation isn’t part of the title (i.e., the punctuation is part of the sentence containing the title), you shouldn’t include in the italics:
Find this useful?
Subscribe to our newsletter and get writing tips from our editors straight to your inbox.
Summary: Writing Book Titles in Essays
We hope you’ll now feel confident when you’re writing and formatting book titles in your essays. Generally, you should set the title in italics when it’s in running text. Remember, though, to check your style guide. While the standards we’ve covered are the most common, some style guides have different requirements.
And once you finish writing your paper, make sure you send it our way! We’ll make sure any titles are formatted correctly as well as checking your work for grammar, spelling, punctuation, referencing, and more. Submit a free sample to try our service today.
Frequently Asked Questions
How do you write the title of a book in a sentence.
Set the title of the book in italics unless the book is part of a larger work (e.g., a book that’s part of a series):
When do you use quotation marks for titles?
Place titles of shorter works or pieces that are contained in a larger work in quotation marks:
Share this article:
Post A New Comment
Got content that needs a quick turnaround? Let us polish your work. Explore our editorial business services.
9-minute read
How to Use Infographics to Boost Your Presentation
Is your content getting noticed? Capturing and maintaining an audience’s attention is a challenge when...
8-minute read
Why Interactive PDFs Are Better for Engagement
Are you looking to enhance engagement and captivate your audience through your professional documents? Interactive...
7-minute read
Seven Key Strategies for Voice Search Optimization
Voice search optimization is rapidly shaping the digital landscape, requiring content professionals to adapt their...
4-minute read
Five Creative Ways to Showcase Your Digital Portfolio
Are you a creative freelancer looking to make a lasting impression on potential clients or...
How to Ace Slack Messaging for Contractors and Freelancers
Effective professional communication is an important skill for contractors and freelancers navigating remote work environments....
How to Insert a Text Box in a Google Doc
Google Docs is a powerful collaborative tool, and mastering its features can significantly enhance your...

Make sure your writing is the best it can be with our expert English proofreading and editing.
How to Write a Book Title in an Essay (MLA, APA etc.)
Formatting your essay correctly ensures that you get full recognition for the hard work you put into it. Wondering what to do? There are two scenarios that lead you to the question of "how to write a book title in an essay":
- You have not been required to use a particular style guide, in which case consistency remains important.
- You have been instructed to use a particular style guide. You now simply need to ensure that you are familiar with its rules.
Regardless of which of these scenarios holds true for you, this guide is here to help.
How to Write a Book Title in an Essay
Many style manuals call on writers use title case and italics to format a book title. Title case rules vary slightly from one style guide to the next, but generally capitalize all important words — nouns, pronouns, verbs, and adverbs. Conjunctions and prepositions are not capitalized unless they are very long (generally more than four letters) or they appear at the beginning or end of a book title.
Writers who are not required to work with a specific style manual can't go wrong if they stick to this style. Some examples would be:
- To Kill a Mockingbird by Harper Lee
- The Gift of Fear and Other Survival Signals That Protect us From Violence by Gavin de Becker
- The Cat With a Feathery Tail and Other Stories by Enid Blyton
If, on the other hand, you're required to use a style guide, it will likely be one of these:
- MLA, commonly used in disciplines relating to literature and social sciences.
- APA, commonly used in psychology and other sciences.
- Chicago, often used in the publishing industry.
- Harvard style, commonly used in philosophy and social sciences.
These are certainly not the only "big players" in the style guide world, but they're ones it's good to be familiar with. There is overlap between these styles, but there are also major differences — so knowing one definitely does not mean you know the others, too.
Guidelines for Writing a Book Title in an Essay
Looking for a short and sharp answer, so you can get on with the rest of your essay? This is it.
| Writing Style / Format | General Rules of Writing a Book Title |
| MLA | Italicize the full title of a book and place it in title case (Conrad, Joseph. ). Place the name of a single chapter in quote marks, instead ("The Great Towns" from by Friedrich Engels). |
| APA | Italicize the book title. Capitalize the first letter, the first letter of a subtitle, and proper nouns. Example: Chapters are placed in title case, but neither italicized nor placed in quote marks. |
| Chicago | Italicize the full title and use title case: by Jonathan Swift. Book chapters are placed in quote marks, and use title case, as with MLA. |
| Harvard | The book title is italicized and placed in title case: by Harper Lee. Chapters are placed in single quote marks: 'Rat' from . |
This quick guide will help you reference the book title of your choosing in the body of your essay, but what about your Works Cited pages? Each style guide offers different rules, and we'll use the same book as an example to illustrate the differences.
- MLA uses the following format: Author Last Name, First Name. Title of Book . City of Publication, Publisher, Publication Year. Example: Card, Orson Scott. Ender's Game. Tor Books, 1985. (You only have to detail the city of publication if the book was published before 1900, the publisher has offices in many localities, or the publisher is not known in the US.)
- APA uses the following format: Author Last Name, First Name. (Year of Publication). Title of book. Example: Card, Orson Scott. (1985). Ender's game.
- Chicago style uses the following format: Author Last Name, First Name. Book Title: Subtitle . Place of publication: Publisher, Year. Example: Card, Orson Scott. Ender's Game . Tor Books, 1985.
- Harvard uses the following format: Author Last Name, First Initial. (Publication Year). Title . ed. City: Publisher. Example: Card, O. (1985). Ender's Game. Tor Books.
If, after researching, you cannot find relevant information about publication years, publishers, or the city in which a book was published, you may omit it. For a full guide, it is always best to have a physical copy of the latest edition of the style manual you are using. You can, however, get by without this if you need to.
Should you still not know what to do, it will be helpful for you to know that you can "generate" citations for a particular style manual with the help of online tools like Cite Me . These are not always accurate, so if you decide to use one, always check the citation manually.
Why Is Proper Formatting Important?
All of the well-known style manuals ultimately serve the very same set of purposes, although they were each developed for a particular niche. The goals of these style manuals are both explicit and implicit:
- Following a style guide ensures consistency throughout a document, in this case an essay.
- Consistency ensures that reader's understand precisely what the writer is talking about, without exerting any effort on figuring that out. Clarity is especially important in academic writing.
- By using a style guide within a certain discipline, you show that you understand the rules within that discipline. This adds credibility to your voice as a writer. You have done your homework, have ideally bought the style manual, and are part of the "in group".
- Sticking to a certain style guide makes it easier for relevant parties to check your references, which they can then use to perform further research.
Students are increasingly asked to refer to style guides at all levels, including in high school. In this case, formatting your essay correctly, in accordance with the right style manual, serves two additional purposes:
- You'll lose points if you don't do it right, offering you an additional reason to do your research.
- Getting used to these formats prepares you for further education. If you are in high school, it prepares you for college-level writing. If you are an undergraduate student, it prepares you for academic work at the graduate and post-graduate levels.
Can you start an essay with a book title?
Yes, you can start an essay with a book title. This is a valid stylistic choice, but you will always want to consider your introduction carefully.
How do you write a book title in handwriting?
Students sometimes ask whether it is acceptable to underline book titles instead of italicizing them. This practice indeed stems from a time in which most students wrote their essays by hand. Although it has largely fallen out of practice now, you can still underline a book title if you are handwriting your essay.
How do you write a book title and chapter in an essay?
You should mention the chapter title first: "Rat" from Ender's Game by Orson Scott Card. Consult the relevant style manual to ensure you get the formatting right.
Can you shorten a book title in an essay?
Yes, you can. Reference the full title the first time you mention it (for example: Furiously Happy: A Funny Book About Horrible Things ). The next time you mention the book, you may simply refer to Furiously Happy .
Related posts:
- How to Write the Date in MLA Format
- How To Write A Movie Title In An Essay
- Someone Walked Over My Grave - Meaning and Origin
- 14 Tips to Help you Write An Essay Fast
- Go Pound Sand - Meaning, Usage and Origin
- How to Write a DBQ (APUSH) Essay?
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *

How To Write Book Titles The Proper Way: A Complete Guide For Writers
- February 10, 2022
Book titles within essays or papers can be tricky. There are specific rules that are given for how to include a book title in a way that sets it apart from the content of your writing given by the Modern Language Association. However, as with many other things in life, there are exceptions to the rules. This article will guide you through the rules of the writing style guides so that you can include a book’s title in your paper or essay correctly.
How to write book titles:
Style guides and book titles.
When it comes to book titles within text, there are a few different style guides that have rules you can follow, depending on your writing type. The three types that you will encounter most often are; MLA style, Chicago manual of style, and APA. A writing instructor will usually tell you what style guide you are expected to use for a particular essay or paper.
MLA Style Guide
The MLA handbook states that you should always italicize book titles when styling book titles within your text. The exception to this rule are religious texts. You would not italicize the Holy Bible or the sacred books or titles of other religions. Note the following example.
Pam had stayed most of the summer indoors, re-reading her favorite book series. She was already up to Harry Potter and the Philosopher’s Stone , and she didn’t regret not being more active or going outside.
In the above example, the book title is italicized. Fiction titles and nonfiction titles alike must be in italics when within the text.
Series Titles in MLA
In the above example, a book from a series was used. But what if the text had not specified which book from the series Pam was reading? Would it still need to be in italics? The answer is: in this case, yes. In other cases, sometimes.
It’s really not as confusing as it seems. When you are talking about a book series but don’t want or need to include the complete series titles for the purposes of your work, you only have to put words in italics that also appear in the book titles. So, because Harry Potter is part of the title of all of the books in the series, you would italicize his name every time you mention the book.
However, if you were talking about Katniss Everdeen, you would not have to do this, as the book series she is featured in doesn’t use her name in the titles of The Hunger Games series. The same would be true of books like the Nancy Drew books.
Quotation Marks
There are instances in which titles should be placed inside of quotation marks within a paper or essay. This is done when you cite the titles of poems , a chapter title, short stories, articles, or blogs.

So, for example, if you were to write a paper that featured a poem from a book, you would put the book title in italics and the poems cited in quotation marks.
An example of an enduring love poem is “Annabel Lee” from The Complete Tales and Poems of Edgar Allan Poe.
Chapter Title
Another time that quotation marks should be used is when using the title of a chapter. If you are citing a specific chapter of a book, you would enclose the title of the chapter in quotation marks, and the title of the book should be in italics.
The desperation and sadness of a man on death row can be seen in the “Wild Wind Blowing” chapter of Norman Mailer’s The Executioner’s Song.
Short Stories
Short stories are another case. Much like the title of a chapter or poem, in which the title is placed in quotation marks, while the title of the book or collection it is found in is italics. The same can be said for sections, stories, or chapters cited within a literary journal.
Stepping away from his norm of horror and gore, Stephen King writes of trust, love, and regret in his story “The Last Rung on the Ladder,” which can be found in his short story collection Night Shift.
Punctuation Marks
If you are citing a story or title that includes question marks, you need to make sure to italicize the question mark when citing. Keep all punctuation, such as a question mark, comma, ellipses, colon, or exclamation mark, as it is in the original individual books.
If you want a funny and irreverent read, you’ve got to try Are You There, Vodka? It’s Me, Chelsea. Chelsea Handler has done a phenomenal job of being vulgar, relatable, and explaining life from her viewpoint in this hilarious and memorable book.
The Digital Age: Are Book Titles Underlined Anymore?
MLA style used to dictate that a book title should either be in italics or underlined. However, that is no longer the case. As computers started to take over as the major tool used in writing, it became unpopular to underline book titles. Therefore, this rule was dropped from the style guides.
However, it should be mentioned that when handwriting an essay or research paper, many instructors prefer that you underline book titles, as it’s relatively difficult to handwrite italics. If you are in a writing course or a class that is heavy on handwritten work, be sure to ask your instructor or teacher which method they prefer for citing a book title.

How to Come Up with Book Title Ideas
Now that quotation marks, italics, and style guides have been discussed, let’s move on to how you can come up with your own book title. If you’d like a title for your book that sounds interesting and will get a reader’s attention, you may find this article helpful.
Coming up with a good title for your book is a challenging yet essential marketing decision . The right title can make your target audience choose your new book off of the shelf instead of another writer’s work. Your book cover and your book title are quite possibly the most important marketing decisions you will make.
How to Choose a Good Book Title
Certain criteria should be met if you want to have a good book title , and there are specific steps involved in getting there. You may have assumed up until now that titles of books were just spur of the moment decisions made by authors or publishers, but a lot of work goes into writing good titles.
Grab the Reader’s Attention
As a general rule, you want your reader to remember your title and to sound interesting, even without the reader having seen the cover. There are several ways to do this. You can be a little dark with your title, be controversial, provoke the reader, or even be funny.
There are many examples of such works that use memorable and attention-seeking titles. The following are some different titles that are effective and would most likely provoke a reader to grab them from a shelf for closer inspection.
- Burn After Writing (Sharon Jones)
- Love in the Time of Cholera (Gabriel Garcia Marquez)
- Is Everyone Hanging Out Without Me? (Mindy Kaling)
- Are You There, Vodka? It’s Me, Chelsea (Chelsea Handler)
- The Devil Wears Prada (Lauren Weisberger)
- Chicken Soup for the Soul (various authors)
- God Bless You, Dr. Kevorkian (Kurt Vonnegut)
Shorter Titles
If your full title for your book is long, you may end up boring a reader or creating a situation where a reader tries to remember the title of your book, but it’s too long and ends up getting it confused with another book. Although you should always do your best to make sure that there aren’t books by other authors that share a title or have a title similar to your book (more on that in a minute), you don’t want a person to get confused and get the wrong book instead.
Research Your Title Ideas
It’s a good idea to take the titles you have considered for your book and make a list. Then, do your homework. You can use tools like Google Adwords to test out your title to see if there are others like it, or you can simply use any search engine and plug your title ideas into the search bar and see what similar or exact titles of the same words pop up.
Readers are generally busy people. They don’t have the time or the energy to ensure that writers get a title right. They’ll look for the book they are interested in, and if it proves to be too difficult, or if there are other books written that have the same title, they’ll move on to something else.
A writer really has to make sure that they have a title that isn’t going to be ignored, is interesting, isn’t too long, and isn’t too similar to other works.
The same goes for titles of short works within a larger body of work. Short works, like poems or stories, need to have unique titles as well when included in a larger body of work, such as a collection. If stories are similar in nature, be sure to title them differently so that readers will be able to tell them apart, as well.
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Sign up to our newsletter!
Related articles

120 Motivational Quotes About Writing To Inspire A New Writer Like You

How To Register A Kindle On Amazon To Enjoy Your Ebooks In 4 Easy Ways

How To Market A Self-Published Book And Be Profitable In 9 Easy Ways
Book Titles in Essays: Formatting Rules and Examples
How do you write the title of a book in an essay?
A short answer: You look at the assignment’s requirements, see the citation style you should use, and go to a corresponding manual to see what rules it prescribes for writing book titles.
That’s when you might hit a snag:
Most rules for the main styles — APA, MLA, and Chicago — seem identical at first glance. It’s easy to miss a preposition or punctuation rule, capitalize a wrong word, or forget about italics. The devil is in the details, and the final grade for your paper depends on them.
Why not gather the formatting rules for all the citation styles in one place so that it’s more comfortable to compare them and spot specifics?
We’ve got you covered:
In this article, our essay writers share the guidelines for citing book titles in five styles. You’ll see how to write a book title in an essay and how to introduce authors. For the sake of clarity, examples are also here.
What is the title of a book in an essay?
You have several options for formatting a book title in your essay.
First, you can mention it in the essay’s body if you are quoting or paraphrasing information from the book. Also, when compiling a bibliography of the resources you used for research, you’ll need book titles for the reference list.
A book’s title and the details of its author are also essential components in the structure of book review . You’ll mention it in the introduction before summarizing a book’s plot, characters, and themes.
How to put book title in essay:
- Use italics
- Don’t underline or use quotation marks, please
- Don’t capitalize minor words like prepositions and conjunctions of three or fewer letters ( a, of, to, the, etc.) unless they are the first or last word in a book’s title
How to write a book title and author in an essay?
Details to consider:
- Is it an in-text mention or part of a reference list?
- Are you writing about an entire book or one of its chapters?
- Does the book have one or several authors?
- Does the book have a subtitle?
- Is it an independent publication or a collection of essays, series, or short stories? Are you introducing a poem in your essay?
The answers to these questions will give you a clear understanding of how to write a book title and author in an essay. The formatting rules will depend on the above factors and the citation style you should follow. (We’ve covered the two main styles — APA and MLA — in our essay writing book , available on Amazon.)
There are also some general rules to remember, regardless of the style. Let’s move to them and explore the principles of citing book titles inside and out.
How to Introduce a Book in an Essay: General Rules
Here’s what all the styles agree on in terms of how to introduce a book in an essay:
1 — Italicize the titles of self-contained books. If you mention a novel, a movie, a stand-alone poem, a play, a database, or a website, there’s no need to use quotation marks. For example:
- Harry Potter by J.K. Rowling
- Shakespeare’s Romeo and Juliet
- If by Rudyard Kipling
2 — The titles of parts within a book should go in quotation marks: chapter titles, titles of poems inside a collection, acts or scenes in a play, and so on. For example:
- The Great Gatsby’s “Chapter 5: The Meeting”
- “The Mirror of Erised” from Harry Potter and the Philosopher’s Stone
3 — Capitalize both stand-alone book titles and the parts within a complete work. For example:
- The Dark Tower: The Gunslinger by Stephen King
- “Sometimes They Come Back” from Stephen King’s Night Shift
4 — When the title of a book goes within another title (like in cases with monographs about novels or poems), you should also use italics for independent works and single quotation marks for short stories and parts of books.
For example, this is how to write the title of a journal article containing the book’s title:
- “The Unbearable Weight of Authenticity: Zora Neale Hurston’s Their Eyes Were Watching God and a Theory of Touristic Reading.”
And this is how you’d write a journal article title containing the title of a short story:
- “Individualism in O’Connor’s ‘A Good Man is Hard to Find.'”
When to use a capital letter is the trickiest part of writing book titles in essays. The rules vary between style guides and their editions, which can appear confusing and make it more challenging for students to align with the requirements and ensure consistency.
Below, we’ll explore how to put book title in essay according to five different citation styles: APA, MLA, Chicago, CSE, and AMA.
How to Write the Title of a Book in an Essay: Citation Styles
While most students use APA and MLA citation styles in their academic papers, some institutions also assign alternatives like AMA or CSE. We’ve chosen the five most widespread styles for this guide so that you can have all the rules in one place and see the tiny differences between them for more precise writing.
Here, you’ll find the book title writing guidelines for these styles:
- APA (the American Psychological Association)
- MLA (the Modern Language Association)
- Chicago, aka CMOS (the Chicago Manual of Style)
- CSE (the Council of Science Editors)
- AMA (the American Medical Association)
We also recommend using an AI essay checker to revise your papers and reference lists once your drafts are ready. Whatever style you use to cite sources, this will help ensure that your text doesn’t look AI-generated. (Believe us, your teachers won’t appreciate it.)
APA is the documentation style that the American Psychological Association uses for citing sources. Originated in 1929, this form of writing is standard for social sciences like psychology, communications, sociology, and anthropology. Sometimes, it also relates to engineering, nursing, education, and other corresponding fields.
APA addresses manuscripts for journals and the academic papers students write in college. It’s the most popular and common citation style for the essays your teachers will assign during a course.
The latest version is APA Style’s 7th edition, released in 2020.
When it comes to formatting the title of a book in an essay, APA style’s requirements are easy to remember. Take a look:
| Write the title in italicsDo not use quotation marks (unless you’re speaking about the book’s chapter, not the entire piece)Capitalize the first and last words, proper names, and all words of four or more letters ( etc.)Capitalize words that appear after punctuation marks (colons, semicolons, em dashes, etc.), even if it’s an article or a short prepositionCapitalize the second part of hyphenated wordsDo not capitalize articles ( ) or prepositions/conjunctions of three or fewer letters unless they come first or last Examples: | Start with the last name, followed by the initials and separated with a commaIf a book has several authors, enumerate them alphabetically; use “&” before the last author in the listIf it’s an edited work, use the editor’s last name and initials and add “Ed.” In the case of several editors, enumerate them alphabetically and add “Eds.” after the namesIf the work has both an author and an editor, place the author in the beginning and add the editor’s name in brackets after the book titleIn the case of a corporate author, write the organization’s name in full Examples: Kulish, M.Fitzgerald, F. Scott, Hemingway, E., & Vonnegut K.Black S. . (White A. & Brown L., Eds.)American Psychological Association |
MLA is a citation style created by the Modern Language Association and is mainly used in humanities like linguistics, literature, philosophy, and cultural and media studies. It’s the second most used style (after APA), with the most recent manual released in 2021 (the 9th edition).
The manual focuses on the formatting rules for in-text citations, which most users find challenging. It also has expanded guidelines on research papers, grammar mechanics, and inclusive language.
Here’s how to write a book title in an essay, according to MLA:
| Write the title in italicsDo not use quotation marks (unless you’re speaking about the book’s chapter, not the entire piece)Capitalize the first and last words, proper names, all significant words, and subordinating conjunctions ( etc.)Do not capitalize articles ( ), prepositions (unless they come first or last), or coordinating conjunctions ( etc.) Examples: | Start with the last name, followed by the first name and separated with a commaIf a book has several authors, enumerate them like on the title page: Use the last-first-name system for the first author and then name the others in the usual name-surname order. Place “and” before the last author in the listIf there’s a corporate author, use the organization’s name Examples: Yohansen, MaikKing, Stephen, and Owen KingModern Language Association |
The Chicago Manual of Style (CMOS) is more common for published works than college papers. Many see it as the top one for writers, editors, and publishers to follow when formatting content. Unlike APA or MLA, Chicago style provides two methods for documenting sources:
- Author-date , recommended for works in the physical, natural, and social sciences. It requires using parenthetical citations in the text, with a corresponding entry on the reference page.
- Notes-bibliography , recommended for works in humanities and some social sciences. It requires using numbered footnotes in the text, with a corresponding shortened citation at the bottom of the page and a fuller citation on the reference page.
The author-date system is similar to APA style and, thus, more common for college essays. When in-text, you mention the author, the date, and the page number (if applicable) in parentheses after the quotation. Like this:
- Enlightenment thinkers, such as Kant, believed in the “universal, eternal, and … immutable qualities of all of humanity” (Harvey 1990, 12).
We can almost hear you asking:
“Can you write my essay in this format?”
Yes, we can. Whenever necessary, ask our academic expert for help with your written assignments. When asking your question, provide detailed requirements, including the citation style you need, so that they know what formatting rules to follow.
Below, let’s explore how to put a book title in an essay in CMOS:
| Write the title in italicsDo not use quotation marks (unless you’re speaking about the book’s chapter, not the entire piece)Capitalize the first and last words, proper names, and all significant wordsDo not capitalize articles ( ), prepositions, or conjunctions (regardless of their length) unless they are the first or the last words of the title or come after a colon Examples: | Start with the last name, followed by the first name and separated with a commaIf a book has several authors, enumerate them like on the title page: Use the last-first-name system for the first author and then name the others in the usual name-surname order. Place “and” before the last author in the listIf there’s a corporate author, use the organization’s name Examples: Bahrianyi, IvanGolding, William, and Harper LeeUniversity of Chicago Press |
Previously known as CBE (the Council of Biology Editors), this style provided formatting guidelines for the editors of biology journals. Today, we know it as CSE (the Council of Science Editors), and it includes many scientific fields in the life sciences, the physical sciences, and mathematics.
As with CMOS, CSE style recommends two systems for documenting sources:
- Citation-sequence , listing sources on a reference page according to the order of their appearance in the document.
- Name-year , which is similar to the author-date system used in Chicago and APA.
The complete guide is available in Scientific Style and Format: The CSE Manual for Authors, Editors, and Publishers (8th ed.) by the Council of Science Editors. Below, we’ll explore how to write a book title in an essay according to this citation style.
| Do not use italics, underlines, or quotation marks for book titlesUse a sentence case; only capitalize the first word in the title, proper names, acronyms, and initials Examples: Plant cell culture: essential methodsThe man who loved childrenThe bridge of San Luis Rey | Start with the last name, followed by the initials and with no commas or periods between themIf a book has several authors, enumerate them like on the title page; use “&” before the last author in the listIf there’s a corporate author, use the organization’s name Examples: Salinger JDMoore A, Tolkien JRR, & Woolf VCouncil of Science Editors |
AMA stands for the American Medical Association, so it’s a standard citation style in medicine. While it’s less popular than APA or MLA, we’ve decided to include it in this guide anyway, given that medical students might find it helpful.
Is AMA citation the same as APA?
Not quite. While sharing some nuances, the core difference between these two citation styles is that AMA doesn’t use an author-date system in the text. Instead, we use a superscript numbering system here. Like this:
- “Smith² argues that….”
Also, unlike APA, AMA style doesn’t organize the reference list alphabetically, but numerically, based upon the order of the sources’ appearances in the text.
How to write the title of a book in an essay when you use AMA style:
| Write book titles in italicsCapitalize all significant words, including two-letter verbs like “be” or “is”For book chapters, only capitalize the first words, proper names, and abbreviations that you’d typically capitalizeDo not use quotation marks Examples: | Start with the last name, followed by the initials and with no commas or periods between themIf a book has several authors, enumerate them like on the title page; use “&” before the last author in the listIf there’s a corporate author, use the organization’s name Examples: Fitzgerald FSBahrianyi I, Khvylovy M, & Pidmohylny VAmerican Medical Association |
How to Format a Book Title in an Essay
Long story short, most citation styles agree on using the same format for book titles in essays: capitalized, italicized, and with no underlining or quotation mark (unless you write about a book’s chapter or a shorter work like an article, an essay, or a poem within a more extensive work).
Speaking of underlined titles:
When googling information on how to write a book title in an essay, you can find questions from people wondering if they need to underline titles in papers. It’s an old-time practice from when essays were written by hand: You can’t italicize when handwriting, so you underline a title to distinguish it.
Check any book review sample online, and you’ll see that underlining isn’t a common practice anymore.
How to format a book title in an essay in your reference list:
| Last name, Initials. (Year of Publishing). Publisher. | King, S. (2019). Scribner. | |
| Last name, First Name. . Publisher. Year of publication. | King, Stephen. Scribner. 2000. | |
| Last name, First Name. . Publishing place: Publisher. Year of publication. | King, Stephen. New York City (NY): Viking. 1989. | |
| Last name Initials. Year of publishing. Book title. Edition. Place of publication: publisher. | Schott J. 2002. Leading antenatal classes: a practical guide. 2nd ed. Boston (MA): Books for Midwives. | |
| Last name Initials. Publisher; Year of publication. | Gallagher EB. . Temple University Press; 1993. |
So, How Do You Write the Title of a Book in an Essay?
Now that you’ve read our detailed guide on how to write a book title in an essay, let’s recap:
- Read the guidelines from your teacher before writing: What citation style do you need to follow?
- Check the manual for your assigned style (APA, MLA, or any other) to ensure that you format the book titles and author names correctly.
- Most citation styles (except for CSE) tell you to italicize and capitalize book titles in essays. Nevertheless, proofread carefully to avoid mistakes with the formatting of prepositions, punctuation, and subtitles.
Are you looking for a title for your next paper? Get help from our essay title generator : Give it several keywords on your topic, and get relevant and creative titles that hook your readers.
Photo by Thought Catalog from Unsplash
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
What our customers say
Our website uses secure cookies. More details
Get professional help from best writers right from your phone

Grab our 3 e-books bundle for $27 FREE
How to Write a Book Title in an Essay in MLA & APA Styles
Learn how to properly cite book titles in your essays using MLA & APA styles. Discover guidelines for writing book titles in essays with ease.

Rijvi Ahmed
Last updated on Mar 14th, 2024

When you click on affiliate links on QuillMuse.com and make a purchase, you won’t pay a penny more, but we’ll get a small commission—this helps us keep up with publishing valuable content on QuillMuse. Read More .
Table of Contents
In academic writing, attention to detail is paramount, especially when it comes to writing essays. An often overlooked aspect is how to properly incorporate book titles into text, a seemingly simple thing that can lead to confusion if the task is not done properly.
Whether you follow the Modern Language Association (MLA) or American Psychological Association (APA) approach, an understanding of how to write a book title and how a book’s title is structured is essential to the clarity and coherence of your writing.
In this guide, we will explore the complexities of a book title for inclusion in an essay according to the MLA-APA method. By the end, you will have a clear understanding of the guidelines for each process along with practical writing tips for ensuring that your essays meet proper editorial standards.
So let’s embark on this journey to demystify the process of writing book titles in essays, and ensure that your academic writing shines with professionalism and accuracy.
Importance of Properly Formatting Book Titles in Essays
Properly organizing book titles into essays is a seemingly mundane part of academic writing, but its importance cannot be overstated. Every aspect, from enhancing readability to supporting scholarly integrity, emphasizes the central role of formalization in academic discourse.
By adhering to established formatting guidelines, whether MLA, APA, or other academic styles, authors ensure a clear and cohesive presentation of their work, allowing readers to focus on the content rather than being swallowed up by inconsistent policy
Properly formatted book titles serve as signposts, guiding readers to the sources of information and ideas, thereby facilitating further exploration and engagement with the scholarly conversation.
By maintaining these standards, writers uphold the principles of academic honesty and integrity, protecting the credibility of their work and contributing to the advancement of knowledge in their respective fields.
From increasing readability and clarity to supporting and inclusive scholarly integrity, each piece emphasizes the critical role of coherent organization in academic discourse. Through organizational guidance that is established in compliance, writers not only demonstrate their attention to detail and commitment to professionalism.
General Rules When Writing a Book Title in an Essay
When incorporating a book title into an essay, whether you’re following MLA, APA, or another citation style, there are general rules to adhere to for clarity and consistency. Here are some overarching guidelines:
1. Italicization vs. Quotation Marks : Determine whether the citation style you’re using requires book titles to be italicized or enclosed in double quotation marks. In MLA style, for instance, book titles are italicized, while in APA style, they are enclosed in double quotation marks. Be sure to follow the specific requirements of your chosen citation style.
2. Punctuation : Regardless of the citation style, book titles should be punctuated properly. This means using appropriate punctuation marks such as commas, periods, question marks, or exclamation points within or after the title, depending on the context of your sentence.
3. Capitalization : Follow the capitalization rules prescribed by your citation style. Typically, capitalize the first word of the title, as well as any proper nouns or adjectives. However, lowercase all other words unless they are proper nouns or adjectives.
4. Consistency : Maintain consistency throughout your essay in how you format book titles. Whether italicized or enclosed in quotation marks, ensure that you apply the chosen formatting consistently each time you reference a book title within your text.
5. In-text Citation : Provide an in-text citation whenever you reference a book title within your essay. This citation typically includes the author’s last name and the publication year, enclosed in parentheses. Consult the guidelines of your citation style for specific formatting requirements for in-text citations.
6. Reference List or Works Cited : At the end of your essay, include a reference list (APA) or works cited page (MLA) that provides full bibliographic details for all sources cited in your essay, including book titles. Format the entry for each book title according to the guidelines of your chosen citation style.
7. Accuracy : Double-check the spelling and formatting of book titles to ensure accuracy. Incorrectly formatted titles or typographical writing errors can detract from the professionalism and credibility of your essay.
By following these general rules, you can effectively integrate book titles into your essay while maintaining clarity, consistency, and adherence to the conventions of your chosen citation style.
What Are MLA & APA Styles
Two well-known citation formats used in academic writing are MLA (Modern Language Association) and APA (American Psychological Association). Both styles provide guidelines for formatting various elements of a paper, including citations, references, and formatting of titles, such as book titles within essays.
In MLA style, book titles are generally italicized within the body of the essay. This means that when referring to a book title within the text, it should be italicized to distinguish it from the surrounding text. Additionally, MLA style typically requires authors’ names and page numbers to be included in in-text citations for direct quotations or paraphrased information.
On the other hand, APA style follows slightly different conventions for formatting book titles in essays. According to Wikipedia, APA style (also known as APA format) is a writing style and format for academic documents such as scholarly journal articles and books. In APA style, book titles are not italicized; instead, they are enclosed in quotation marks. Similarly to MLA style, APA requires authors’ names and publication years to be included in in-text citations for direct quotations or paraphrased information.
Understanding these differences is essential for properly formatting book titles in essays according to MLA and APA styles. While both styles aim to maintain consistency and clarity in academic writing, they have distinct rules regarding the formatting of book titles.
Adhering to the specific guidelines of each style ensures that your writing meets the expectations of scholarly standards and effectively communicates your ideas to readers.
How to Write a Book Title in an Essay in MLA Style

Writing a book title in an essay in MLA style requires attention to detail and adherence to specific guidelines to maintain consistency and accuracy. Whether you’re discussing a classic novel, a contemporary work of fiction, or a scholarly publication, correctly formatting the book title is essential for conveying your ideas effectively. Let’s explore the steps for properly formatting a book title in an essay according to MLA style:
1. Italicize the Title : One of the fundamental rules in MLA style is to italicize the title of the book when mentioned within the body of the essay. Italicization serves to differentiate the title from the surrounding text and emphasizes its importance to the reader. For instance:
– In “To Kill a Mockingbird,” Harper Lee explores themes of racial injustice and moral growth.
2. Use Title Case : When writing the title of the book, capitalize the principal words, including nouns, pronouns, verbs, adverbs, and adjectives. Articles, conjunctions, and prepositions are generally not capitalized unless they are the first or last word in the title or part of a hyphenated word. Here’s an example:
– “The Catcher in the Rye” remains a classic coming-of-age novel.
3. Include Author’s Name : It is customary to include the author’s name when introducing the title of the book in your essay. This provides essential context for the reader and acknowledges the author’s contribution to the work. Typically, the author’s last name is sufficient, especially if it’s clear from the context which work is being referenced. For example:
– In “Beloved” by Morrison, the legacy of slavery haunts the characters’ lives.
4. Format In-Text Citations : When quoting directly from the book or paraphrasing its content, it’s crucial to include an in-text citation following MLA guidelines. The citation should include the author’s last name and the page number(s) from which the quotation or paraphrase is taken. For instance:
– (Hemingway 22) or (Smith and Johnson 45)
5. Titles Within Titles : If the book you’re discussing contains a title within its title, such as a collection of essays or short stories, follow specific formatting rules. Italicize the title of the larger work and enclose the title of the smaller work in double quotation marks. Here’s an example:
– In “The Norton Anthology of English Literature,” the essay “Shakespeare’s Women” examines the portrayal of female characters in his plays.
By adhering to these guidelines, you can effectively integrate book titles into your essays under MLA style. Consistency and accuracy in formatting not only enhance the professionalism of your writing skills but also demonstrate your commitment to scholarly standards and integrity.
How to Write a Book Title in an Essay in APA Style

Writing a book title in an essay according to APA style necessitates adherence to specific formatting conventions to ensure clarity, consistency, and compliance with academic standards. Here’s a comprehensive guide detailing the steps involved:
1. Punctuation and Enclosure : Book titles must be enclosed within double quotation marks. This distinguishes them from other texts in the essay and signals to readers that they are referring to the title of a specific work. For instance, if you’re discussing the book “To Kill a Mockingbird” within your essay, it should be presented as “To Kill a Mockingbird.”
2. Capitalization : When formatting book titles in APA style, capitalize the first word of the title, as well as any proper nouns or adjectives. However, all other words in the title should be lowercase unless they are proper nouns or adjectives. For example, the book title “The Catcher in the Rye” follows this capitalization pattern.
3. Italicization vs. Quotation Marks : Unlike MLA style, which mandates italicization for book titles, APA style requires book titles to be enclosed in double quotation marks. This distinction is crucial for adhering to APA guidelines accurately.
4. In-text Citation : Whenever you reference a book title within your essay, it’s essential to provide an in-text citation to acknowledge the source. This citation typically includes the author’s last name and the publication year in parentheses.
For example, you might write, “In the novel ‘1984’ (Orwell, 1949)…”
5. Reference List Entry : After your essay, you must include a reference list that provides comprehensive bibliographic details for all sources cited in your work. When listing a book in the reference list, include the author’s last name followed by their first initial, the publication year in parentheses, the book title in italics (or within double quotation marks if it’s an article or chapter within a larger work), the publication location, and the publisher’s name. Here’s an example of a book reference list entry:
Orwell, G. (1949). 1984. New York, NY: Harcourt Brace Jovanovich.
By meticulously following these guidelines, you can effectively integrate book titles into your essay according to APA style, ensuring accuracy, professionalism, and adherence to academic conventions.
In conclusion, correctly formatting a book title within an essay is essential for maintaining consistency and adhering to the guidelines set forth by MLA and APA styles. Remember to italicize the title in both styles and to capitalize significant words according to the rules of each style guide.
By following these simple guidelines, writers or authors can ensure their essays are properly formatted, enhancing the overall professionalism and credibility of their work.
So, whether you’re citing a classic novel or a contemporary bestseller, mastering the art of writing book titles in MLA and APA styles will undoubtedly elevate the quality of your writing.
FAQs: How to Write a Book Title in an Essay in MLA & APA Styles
What’s the importance of correctly formatting book titles in essays.
Accurate formatting of book titles is crucial for academic integrity and professionalism in writing. It demonstrates your understanding of citation styles like MLA and APA and enhances the clarity and organization of your essay.
How do I format a book title in MLA style within an essay?
In MLA style, italicize the titles of books and use title case (capitalize the first letter of major words and any important words in the title). For example, “The Great Gatsby” by F. Scott Fitzgerald.
What about formatting book titles in APA style?
In APA style, capitalize only the first word of the title, the first word of the subtitle (if any), and any proper nouns. Additionally, italicize the title. For example, “The Great Gatsby” by F. Scott Fitzgerald.
Are there any exceptions to the italicization rule for book titles?
Yes, if you’re writing by hand or using a typewriter where italics aren’t possible, underline the title instead.
How do I reference a book title in-text using MLA and APA styles?
In MLA style, place the author’s last name and the page number in parentheses after the quote or paraphrase. For example, (Fitzgerald 47). In APA style, include the author’s last name and the publication year, separated by a comma, within parentheses. For example, (Fitzgerald, 1925).
Do I need to include the author’s name in the essay when referring to the book title?
Yes, both MLA and APA styles require you to include the author’s name when referring to the book title in your essay. This helps provide context and credit to the original author.
What should I do if the book title contains a subtitle?
In both MLA and APA styles, include the subtitle after the main title, separated by a colon. Capitalize any proper nouns and the subtitle’s first word. For example, “The Great Gatsby: A Novel of the Jazz Age.”
Can I abbreviate book titles in my essay?
It’s generally recommended to use the full title of the book to ensure clarity and accuracy. Abbreviations might lead to confusion, especially in scholarly writing.
Where can I find more detailed guidelines for formatting book titles in MLA and APA styles?
You can refer to the official MLA Handbook or the Publication Manual of the American Psychological Association for comprehensive guidelines on formatting book titles and other citation-related issues. Additionally, numerous online resources and style guides provide detailed explanations and examples.
How we've reviewed this article
Our content is thoroughly researched and fact-checked using reputable sources. While we aim for precision, we encourage independent verification for complete confidence.
We keep our articles up-to-date regularly to ensure accuracy and relevance as new information becomes available.
- Current Version
- Mar 14th, 2024
- Mar 4th, 2024
Share this article

How to Write an Essay: A Three-Step Guide with Examples
Is the essay deadline making you nervous? Do you ever look at the flashing line on your computer screen and want to write something, but just can’t think of anything and feel stuck? Fear not, a fellow student or maybe even a writer facing a new challenge! This guide is

How to Write an Essay Introduction: A Proven 4-Step Process With Examples
The first impression is everything, which also holds for essays. A well-written introduction is like a captivating trailer for a movie; it hooks the reader, sets the stage for your argument, and makes them eager to delve deeper. But writing a great introduction can be tricky. Don’t worry though! This
In academic writing, attention to detail is paramount, especially when it comes to writing essays. An often overlooked aspect is how to properly incorporate book titles into text, a seemingly simple thing that can lead to confusion if the task is not done properly. Whether you follow the Modern Language
Report this article
Let us know if you notice any incorrect information about this article or if it was copied from others. We will take action against this article ASAP.
- Profile Page
- Edit Profile
- Add New Post
Read our Content Writing Guide .

How to Write Book Titles in Essays: APA, MLA, Chicago Styles
It’s your practical and up-to-point guide on how to write a book title in an essay. You’ll get the formatting rules and examples for citing book and author names in academic papers.
We’ve covered the top three citation styles: APA, Chicago, and MLA.
How to Write the Title of a Book in an Essay
First, remember the general rules of citing book names in academic works.
Here’s how to cite books in essays :
- Use capitalization. Every word of a book’s name goes in the title case, except prepositions, articles, and coordinating conjunctions.
- Use italics for longer and independent works. Use double quotations for shorter ones (poems, articles, book chapters, or play acts and scenes).
- Use single quotations for a book’s title within another title. (When citing monographs about literary works, for example.)
While capitalization rules depend on the citation style, some general tips have a place to be. Please, no capitalization for:
- Articles: a, the (unless the book title begins with it)
- Coordinating conjunctions and prepositions: of, and, or, but, for, to, nor, in, so (unless the book title begins or ends with it)
Subordinating conjunctions (although, unless, because, if) go in capital letters.
How to Write a Book Title in an Essay: APA
| Independent and self-contained books: Book chapters or short works (poems, essays, songs, articles): “Quotation Marks for Names” | In her work, , Simone de Beauvoir explores the concept of women’s oppression. She argues for their liberation from traditional gender roles. My favorite book is “The Order of the Phoenix.” |
APA (American Psychological Association) is the most popular style for citing academic works. It’s common for the social sciences like Education, Psychology, Sociology, and others. The current edition: 7th (2019).
Book titles in APA stand for:
- Italics. (If a book name includes any punctuation, italicize it too.)
- Capitalization. (Capitalize all words longer than four letters , regardless of the part of speech. Also, use capital letters for two-part words and those coming after a dash or a colon.)
- Double quotations instead of italics. (When citing a short work like an article or a poem; when citing a book chapter or when the book is a part of an anthology.)
For example:
The Lord of the Rings but “The Fellowship of the Ring” (The latter is part of the trilogy.)
Related: How to Cite a Movie in APA Format
How to Write the Name of a Book in an Essay: Chicago
| Independent and self-contained books: Book chapters or short works (poems, essays, songs, articles): “Quotation Marks for Names” | In , the author delves into the chilling cat-and-mouse game between a retired detective and a deranged killer, presenting a gripping exploration of the human psyche. In Stephen King’s , the pivotal moment comes in “End of Watch,” bringing the story to a dramatic and suspenseful climax. |
The Chicago Manual of Style is a guide by the University of Chicago. It’s common for fields like History, Fine Arts, and Business. The current edition: 17th (2017).
How to format book titles in Chicago:
- Italicize longer and independent works; put shorter ones in double quotations.
- Use italics for punctuation within a title.
- Capitalize all words except articles (a, the) and ALL prepositions or conjunctions (regardless of length).
For example:
In George Orwell’s 1984 , the author presents a dystopian society characterized by pervasive government surveillance and the suppression of individual freedom. The harrowing events in “Chapter 2,” where Winston Smith begins to rebel against the Party by starting a forbidden diary, mark a pivotal moment in the novel’s exploration of resistance against totalitarianism.
The style resembles the MLA format, but it’s flexible, allowing you to “break the rules if necessary.”
How to Write a Book Title in an Essay: MLA
| Independent and self-contained books: Book chapters or short works (poems, essays, songs, articles): “Quotation Marks for Names” | In his influential work, Harper Lee examines racial injustice in the American South during the 1930s. In , “The Dementor” explores the chilling encounter with these sinister creatures in the wizarding world. |
MLA format stands for the Modern Language Association. It’s common for humanities like Literature, Culture, Linguistics, etc. The current edition: 8th (2016).
How to format books in MLA:
- Italicize all words, including punctuation and those of two parts or going after colons and hyphens.
- Capitalize all words except articles (a, the) , prepositions, and short conjunctions within a book title.
- Use double quotations instead of italics when writing a book chapter or a part of a book series.
In Little Women , Beth March dies in Chapter 40, “The Valley of the Shadow.”
Formatting Book Author Names in Papers
Use the author’s full name (first and last) to format it in your essay for proper credit.
If a book has two authors, use both last names and initials. For works with three or more authors, use the last name of the first one and add “et all.”
No need to italicize author names in papers.
Why Properly Cite Book Titles in Essays
The short answer:
You won’t get a high grade for an essay. Formatting blunders count as mistakes.
The longer answer:
- You prove writing skills and an understanding of the rules in academia.
- Your papers maintain consistency. It’s critical to stick to criteria to prevent confusion. The consistent format for book headings also serves to better scannability and readability.
- You learn to cite different types of references for your future projects.
Do you italicize book titles?
Yes, you put book titles in italics. Please italicize long and stand-alone works: books, movies, webpages, reports, or music albums. Shorter works’ titles (articles, essays, poems, songs, or book chapters) come in quotations. (1)
Do you underline book titles?
Underlining book titles is an outdated practice. Some still use it in handwritten essays, but it’s not a must-follow rule. Neither APA nor MLA (or Chicago) mentions underlining book names in academic papers.
How to use book title capitalization in texts?
Capitalize every word in a book’s title. Exceptions are articles (a, the), prepositions, and short (three or fewer letters) conjunctions in mid-titles.
Are books italicized in all formatting styles?
Yes, book titles come in italics in all styles: APA, MLA, and Chicago. When citing book chapters or a book as a part of a series, use quotation marks instead.
How to write a book author in an essay?
Use the author’s full name when citing their book in your papers. For works with several authors, mention their last names and initials. Unlike book titles, author names come in standard formatting with no italics.
References:
- https://english.csuci.edu/resources/essay-writing-essentials.htm
- Essay samples
- Essay writing
- Writing tips
Recent Posts
- Writing the “Why Should Abortion Be Made Legal” Essay: Sample and Tips
- 3 Examples of Enduring Issue Essays to Write Yours Like a Pro
- Writing Essay on Friendship: 3 Samples to Get Inspired
- How to Structure a Leadership Essay (Samples to Consider)
- What Is Nursing Essay, and How to Write It Like a Pro
📚 Mastering the Art of Writing a Book Title in an Essay
Mastering the art of writing a book title in an essay.

Why is Book Title Formatting Important?
Understanding the rules, common mistakes to avoid, additional resources.

Should You Indent When Starting a New Paragraph in MLA Format?
Share superior formatting, people also asked.

How should you format an essay that includes a title and a subtitle?

How do you write a title in MLA format?

How do you cite the title of a book in an essay?

How should you represent a book title in a handwritten essay?

Is it appropriate to italicize a book title in a paper?

Should a book title be underlined or italicized when writing an essay?

Is it necessary to italicize book titles every time they are mentioned in an essay?

How should a book title be written in a personal note?
Superior formatting articles.

The Power of a Strong Title: How to Write a Book Title in an Essay

Unlocking Creativity: How to Write a Book Title in an Essay for Maximum Engagement
Login to superior formatting.
How to Write a Book Title in MLA Formatting
by Joe Bunting | 2 comments
You're writing a paper for school and suddenly you stop in the middle of the sentence. You have to write a book title, but you don't how to format it. How do you format a book title in MLA style? Good news: you're in the write place (sorry, I had to).
In this post, we'll talk about MLA style and formatting, whether it's appropriate for your project, and most importantly, how to write a book title in MLA style.
What Is MLA?
MLA stands for Modern Language Association, a society primarily based in the United States but with international standing, that has a mission to “strengthen the study and teaching of language and literature”. Founded in the late 1800s by an American novelist and professor, MLA publishes a set of resources used by students and teachers, including the MLA Handbook for Writers of Research Papers .
The MLA handbook is one of the main style manuals for students and scholars in the world, especially for anyone studying literature, film, or theater.
Should You Format Based on MLA Style?
If you're writing a paper for a class in literature, theater, or film, absolutely use MLA style. Outside of that, it depends. Here are the most frequent style guides associated with various disciplines:
- Literature, Film, Theater: MLA
- Psychology: APA
- Science (Physics, Biology, Chemistry): CSE or APA
- Journalism: AP
- Mathematics: AMA
- Publishing: Chicago
You can find a full list of international style guides here .
Now that you know if you should be using MLA style, how do you format a book title with it?
How to Format a Book Title in MLA Style: Example
In MLA style, book titles are italicized, as so:
Henry Thorough argues in Walden that the best life is lived in deliberate simplicity so as to discover what life truly is about.
In fact, most style guides, including MLA and Chicago style, require book titles to be italicized , not underlined.
If the book title has a subtitle, the subtitle should be italicized as well and separated by a colon to be formatted correctly for MLA style, as in:
Natural History of the Intellect: the last lectures of Ralph Waldo Emerson
Should You Underline Book Titles in MLA Style?
If you are using MLA style, you should not underline book titles. Instead, italicize the titles.
However, AP style, the guide used by journalists, suggests putting titles in quotation marks, not italicization.
Still, I wouldn't recommend underlining a book's title. In fact, I couldn't find a single style guide that requires book titles to be underlined, but if you know of one that does, let me know in the comments!
Which style guide do you use most? MLA? Chicago? APA? AP? Or do you just write based on your own rules?! Let me know in the comments .
Let's cement this formatting lesson in our minds by putting it to use right away with the following writing exercise .
What are your favorite books of all time? Write about what you love about them and why they are your favorites for fifteen minutes . Make sure to use the correct formatting for each title!
When your time is up, post your practice in the comments section . And if you post, please be sure to read a few practices by other writers and share your feedback with them.
Happy writing!
Joe Bunting
Joe Bunting is an author and the leader of The Write Practice community. He is also the author of the new book Crowdsourcing Paris , a real life adventure story set in France. It was a #1 New Release on Amazon. Follow him on Instagram (@jhbunting).
Want best-seller coaching? Book Joe here.

how do you format the title if you’re writing on paper and can’t italicize?
When writing by hand, you can underline book titles.
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Submit Comment
Join over 450,000 readers who are saying YES to practice. You’ll also get a free copy of our eBook 14 Prompts :
Popular Resources
Book Writing Tips & Guides Creativity & Inspiration Tips Writing Prompts Grammar & Vocab Resources Best Book Writing Software ProWritingAid Review Writing Teacher Resources Publisher Rocket Review Scrivener Review Gifts for Writers
Books By Our Writers

You've got it! Just us where to send your guide.
Enter your email to get our free 10-step guide to becoming a writer.
You've got it! Just us where to send your book.
Enter your first name and email to get our free book, 14 Prompts.
Want to Get Published?
Enter your email to get our free interactive checklist to writing and publishing a book.
Verify originality of an essay
Get ideas for your paper
Find top study documents
How to write a book title in an essay: essential guidelines for students
Updated 08 Jul 2024
Unlocking the art of seamlessly integrating book titles in essays is a skill every writer should master. Navigating the intricate landscape of various style guides, such as MLA, APA, Harvard, or Chicago, requires a nuanced understanding of formatting rules. Whether you’re a student aiming for precision in academic writing or an aspiring author looking to enhance your literary prowess, this article will guide you through the nuances of how to write a book title in an essay in different citation styles.
Let’s delve into the subtleties and ensure your book details are written and presented with finesse!
General rules
Crafting a polished essay or writing book report involves well-thought-out content and meticulous attention to formatting, especially when writing book title. Understanding the general guidelines across popular citation styles is essential for presenting your literary references coherently. Discover where all styles agree on how to quote books in essays.
- Following formatting requirements, self-contained and independent books, spanning genres like novels, short stories, a collection of poems, and plays, share a common trait. Are book titles italicized? Yes, they are. Consider the following masterpieces as prime examples:
| by Harper Lee; by Shakespeare; by Walt Whitman; by J.R.R. Tolkien. |
- Components within a larger work, such as acts, chapters, scenes, songs, or individual poems, are embraced by quotation marks. In this case, the whole book title in an essay remains italicized. Let’s see some examples:
| by J.K. Rowling; by F. Scott Fitzgerald. |
- When the book's name mentioned within the overarching title is typically formatted in italics, it’s advisable to employ italic letters consistently. So, the overarching title that encompasses the specific book's discussion should also be italicized.
- If the name of book in essay (poem or novel) referenced within the overarching title typically appears within double quotation marks, it’s recommended to enclose it in single quotation marks instead. Besides, ensure the formatting is consistent throughout your text. If the main title is italicized, remember to maintain this style for the entire heading, including the nested title within quotation marks. If it’s challenging, you may pay someone to do my homework to avoid any inconsistencies in your formatting. Let’s see how these two guidelines can be implemented in the following examples:
- Both stand-alone books and subsections within a larger work adhere to title case capitalization, where major words are capitalized. Consider the following examples:
| and Its Enduring Legacy; ; and Ernest Hemingway’s Art of Storytelling; and Ralph Ellison’s Exploration of African American Experience. |
When considering how to write book titles in essays it's essential to note that capitalization rules can vary significantly between style guides, adding a layer of complexity. Therefore, the path you choose for capitalization should align with the specific style requirements, ensuring consistency and adherence to academic standards. Whether following APA, MLA, or another guide, clarity in title presentation is a key element in elevating the overall quality of your handwritten work.

Don't risk a zero grade!
Hire our experts to proofread, polish, and check your work for accuracy. Just $7/page. Zero AI.
How to write a book title in different citation styles: APA, MLA, Chicago, AMA, and CSE
Navigating the proper formatting of book titles in an essay requires understanding the diverse rules prescribed by various style guides. Whether you’re following the guidelines of the Modern Language Association, American Psychological Association, Chicago Manual of Style, or another specific style, each has unique conventions for presenting the book’s details within your text. In this exploration, we delve into the nuances of formatting an essay body containing book names and emphasize the distinct approaches dictated by different styles.
How to write a book title in an essay in APA style? The rules for indicating the names of books and authors are as follows:
Book titles:
- Italicize all the words and punctuation. Do not underline or place them in quotation marks.
- Capitalize the first word of titles of books in papers, the first word after a colon, and all major words. Avoid capitalizing minor words (e.g., articles, prepositions, conjunctions) unless they are the first word of the name or longer than four letters.
- Always place the book title after the author’s name.
Example: The Great Gatsby .
Author names:
- Write the author’s last name followed by their initials without spaces or periods.
- If multiple authors are indicated in your college papers for sale , separate their names with commas and use an ampersand (&) before the last author’s name.
- If the work has a group or corporate author (e.g., an organization), write the name in full.
Example 1 (single author): Smith, J. A.
Example 2 (multiple authors): Smith, J. A., Johnson, M. R., & Brown, P. S.
Example 3 (group author): American Psychological Association.
Should we italicize or quote book titles according to MLA style? Modern Language Association suggests the following guidelines for academic papers:
- Write the book title in essay in italics. Underlining or placing them in quotation marks is not required.
- Capitalize the first and last words of the title, as well as all major words in between. Do not capitalize minor words unless they are the first or last words of the title or come after a colon.
Example: A Good Man Is Hard to Find.
- Write the author’s full name with the last name first, followed by the first name.
- If there are multiple authors, enumerate them in the order they appear on the title page.
- If the work has a corporate author (e.g., an organization), you can use the organization’s name.
Example 1: Fitzgerald, F. Scott.
Example 2: Smith, John, and Mary Johnson.
Example 3: Modern Language Association.
“Do you italicize authors names in Chicago?” you can ask. Let’s speak about this formatting style. The rules listed in the Chicago essay guide prescribe the following:
- Italicize or underline (older tradition) the titles of larger works, including books. It's more common for readers to see italics in modern Chicago style.
- Capitalize the first and last words of the title, along with all major words in between. Do not capitalize minor words unless they are the first or last words of the title or come after a colon.
Example: Murder on the Orient Express .
- Write the author's full name with the last name first, followed by the first name.
- If there are several writers, indicate them in the order they appear on the title page.
Example 1: Ellison, Ralph.
Example 2: Wood, James, and Mary Jane.
Example 3: University of Chicago Press.
Discover how to quote a book in an essay in AMA. The American Medical Association has specific guidelines for citing and formatting. Here are the rules:
- Use italics for the titles of larger works, including books ─ no need to underline or place them in quotation marks.
- Capitalize only the first word of the title and any proper nouns. Do not capitalize the first letter of subsequent words unless they are proper nouns.
Example: The great Gatsby .
- Write the author's last name followed by their initials without spaces or periods.
- If there are multiple authors, use commas to separate them and apply an ampersand (&) before the last author’s name.
- If the work has a group or corporate author, write the name in full.
Example 1: Fitzgerald FS.
Example 2: Smith JA, Johnson MR, & Brown PS.
Example 3: American Medical Association.
The requirements of the Council of Science Editors are similar to the AMA style. If you find them challenging or need clarifications, you may always send us your “ write an essay for me ” request and get support with your formatting anytime. Let’s see the essential guidelines:
- Italicize the title of books in essay (here, we mean larger works, not chapters or articles). Do not underline them, and avoid using quotation marks.
- Use capitalizing for only the first word of the title, proper nouns, and the first word after a colon or em dash.
Example: A tale of two cities.
- Write the author's last name followed by a space and their initials, with no commas or periods between the initials.
- If there are multiple authors, use commas to separate them, and use an ampersand (&) before the last author's name.
Example 1: Dickens C.
Example 2: Clark JB, Doe JM, & Anderson KL.
Example 3: Council of Science Editors.
Final thoughts
In conclusion, navigating the intricacies of writing a book title in an essay is an important skill for any writer. By mastering the diverse formatting rules of style guides, you’ll meet academic standards and infuse your work with professionalism. As you embark on your next writing journey, remember that precision matters.
For personalized assistance and expert guidance in creating argumentative essays, consider EduBirdie as your go-to ally. Elevate your writing experience and achieve excellence with the support of a trusted partner. Take the first step towards impeccable papers ─ choose EduBirdie for your writing success!
Was this helpful?
Thanks for your feedback.

Written by Steven Robinson
Steven Robinson is an academic writing expert with a degree in English literature. His expertise, patient approach, and support empower students to express ideas clearly. On EduBirdie's blog, he provides valuable writing guides on essays, research papers, and other intriguing topics. Enjoys chess in free time.
Related Blog Posts
Diversity essay: effective tips for expressing ideas.
In today's interconnected and rapidly evolving world, the importance of diversity in all its forms cannot be overstated. From classrooms to workpla...
Learn how to write a deductive essay that makes you proud!
Learning how to write a deductive essay may sound like a challenging task. Yet, things become much easier when you master the definition and the ob...
A Guide On How to Write a Critical Thinking Essay
This particular term refers to a type of essay written to discuss a specific idea, voice clip, written piece or a video, using purely one’s ideas, ...
Join our 150K of happy users
- Get original papers written according to your instructions
- Save time for what matters most
Discover top guides, trends, tips and expertise from AIO Writers
How to Write a Book Title in an Essay: A Simple Guide
Julia McCoy

Mastering the art of citation is crucial for academic writing, and one common dilemma writers face is how to write a book title in an essay.
In this blog post, we’ll delve into the ins and outs of citing book titles, exploring different citation styles, and providing practical tips to ensure your essays are not only well-written but also properly referenced.
Whether you’re navigating the nuances of MLA, APA, or Chicago style, we’ve got you covered with clear guidelines and examples to help you confidently write book titles in your next masterpiece.
Let’s get started!
Table Of Contents:
How to write book titles in essays, how to format book citations, writing various types of titles in essays, emphasizing book titles in essays, punctuating and capitalizing book titles, examples of writing book titles in essays, faqs – how to write a book title in an essay.
Writing book titles in essays can be tricky, especially with different style guides like MLA, APA, and Chicago. But don’t worry, I’ve got you covered with some tips and examples on how to quote a book title in your essay.
MLA Style Guide
In MLA style, book titles are italicized, both in the text of your paper and in the Works Cited list.
For example: Toni Morrison’s Beloved is a powerful novel about the lasting impact of slavery.

Similarly, in the style guide of the American Psychological Association, book titles should also be italicized in the text and the reference list.
For instance: In The Catcher in the Rye , Holden Caulfield grapples with the transition from adolescence to adulthood.
Chicago Manual
In Chicago style, book titles are italicized in the text and the bibliography.
Like this: Michael Pollan explores the origins of our food in The Omnivore’s Dilemma: A Natural History of Four Meals .
Regardless of the style guide, there are some general formatting rules to keep in mind.
Titles of books should be underlined or italicized. Titles of stories, essays, and poems are placed in quotation marks.
Refer to the text specifically as a novel, story, essay, memoir, or poem, depending on what it is.
Capitalization Rules
Use capital letters to write the title of a novel.
For example, The Secret Garden by Frances Hodgson Burnett.
Quotation Marks
Titles of stories, essays, and poems are placed in “quotation marks.” This helps differentiate them from longer works like novels or non-fiction books.
Now that we’ve covered the basics, let’s dive into some specifics for different types of titles you might encounter.
Journal Articles
If the book title is part of a larger work, like a journal article, it should be underlined instead of italicized.
Short Stories
Titles of short stories should be placed in quotation marks.
For example: “The Lottery” by Shirley Jackson.
Chapter Titles
When referencing a chapter title, enclose it in quotation marks.
For instance: “The Boy Who Lived” from Harry Potter and the Sorcerer’s Stone .
Article Titles
Article titles, like those found in English-language newspapers or magazines, should also be placed in quotation marks.
For example: “Why We Crave Horror Movies” by Stephen King.
Newspaper Titles
Italicize the names of newspapers, like The New York Times or The Wall Street Journal .
The names of websites should generally be italicized, such as The Huffington Post or BuzzFeed .
Book Series
When referring to a book series as a whole, italicize the name of the series. Individual books within the series should also be italicized.
For example: the Harry Potter series by J.K. Rowling, which includes titles like Harry Potter and the Chamber of Secrets .
Sometimes you want to draw extra attention to a book title in your essay. Here’s how to do it effectively.
When to Italicize
As a general rule, italicize the titles of longer works such as books, edited collections, movies, television series, documentaries, or albums.
When to Use Quotation Marks
Shorter works like poems, articles, book chapters, songs, TV episodes, or other shorter works should be placed in quotation marks.
Exceptions to the Rules
As with any rule, there are exceptions. Some style guides prefer underlining to italics. Others may recommend using quotation marks around the title and italicizing or underlining the name of the newspaper or magazine it appears in.
When in doubt, always check with your instructor or the publication you’re writing for.
Punctuation and capitalization are key when it comes to book titles in essays. Get it wrong, and your writing won’t look as polished.
Using Question Marks
If a book title ends with a question mark or exclamation point, include it in the italics.
For example: Do Androids Dream of Electric Sheep? by Philip K. Dick
In general, capitalize the first word and all major words (nouns, pronouns, verbs, adjectives, adverbs, and some conjunctions).
Don’t capitalize articles, prepositions, or conjunctions unless they’re the first or last word.
Some style guides recommend capitalizing prepositions five letters or longer.
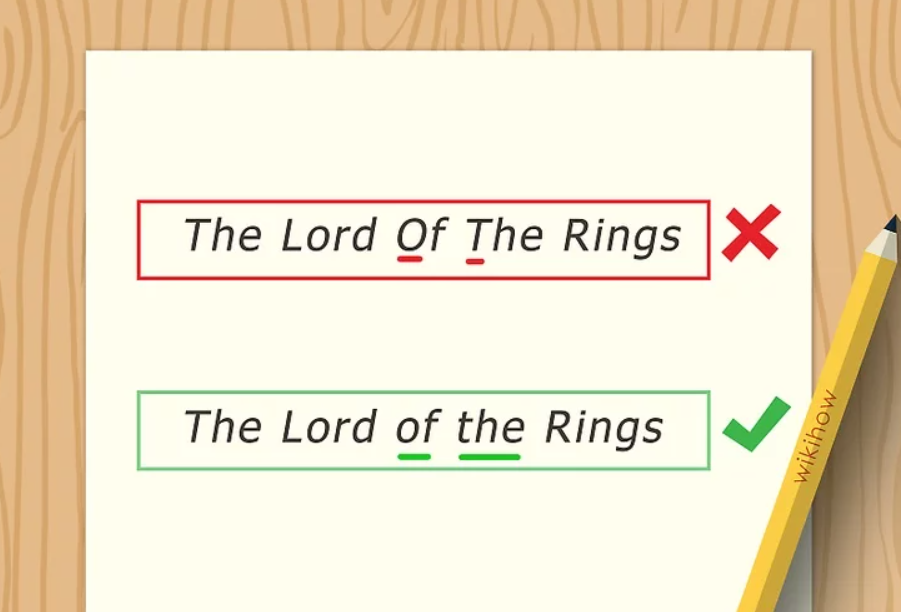
Title case is the most common form of title capitalization and is found in all four major title capitalization styles (AP, APA, MLA, and Chicago).
Capitalize the first word in the title, the last word in the title, and all “major” words in between.
Proper Nouns
Always capitalize proper nouns, such as the names of people, places, organizations, or other proper nouns in a book title.
For example: Harry Potter and the Order of the Phoenix by J.K. Rowling.
Let’s look at some examples of how to write book titles in various situations.
Classic Literature
When referencing a classic work of literature, italicize the book’s title in the text of your paper.
In the Works Cited entry, include the author’s full name, the title of the book (in italics), the publisher, and the year of publication.
For example:
Austen, Jane. Pride and Prejudice . Penguin Classics, 2002.
Contemporary Literature
For a contemporary work, follow the same format in the text of your essay.
In the Works Cited entry, include the author’s name, book title (in italics), publisher, year of publication, and medium of publication (print, web, etc.).
Here’s an example:
Whitehead, Colson. The Underground Railroad . Doubleday, 2016. Print.
Non-Fiction Works
When citing a non-fiction book, use the same format as you would for a fictional work. Italicize the book title in the text and the Works Cited entry. Include the author’s name, book title (in italics), publisher, year of publication, and medium of publication.
For instance:
Krakauer, Jon. Into the Wild . Anchor Books, 1997. Print.
How do you write the title of a book in a sentence?
In sentences, capitalize the first word and proper nouns. If it’s central to your point, italicize it.
Is a book title italicized or in quotes?
Book titles are usually italicized. Quotes are for shorter works like articles or poems.
How do you write a book title in a handwritten essay?
If handwritten, underline book titles instead of using italics to highlight them.
So there you have it – your complete guide to how to write a book title in an essay. By following these simple rules for MLA, APA, and Chicago style, you’ll be able to format your book titles correctly every time.
Remember, the key is to be consistent and pay attention to the details. Whether you’re italicizing, underlining, or using quotation marks, make sure you’re applying the rules consistently throughout your essay.

Written by Julia McCoy

UNLOCK YOUR POTENTIAL
Long headline that highlights value proposition of lead magnet.
Grab a front row seat to our video masterclasses, interviews, case studies, tutorials, and guides.
Experience the power of RankWell®
Grow Your Traffic!
Let's crawl your website and get you some keyword suggestions for your next piece of content.
By continuing, you agree with our Terms and Conditions.
- About Our Blog
- Essay Writing Service
How to Write the Title of a Book in an Essay
- by Lesley V.
- November 21, 2023
If you want to know how to cite books in essays properly, here’s my guide on the top three citation styles.
You’ll learn to cite the title of books in essay in APA, MLA, and Chicago.
Get ready to remember formatting guidelines and consider examples.
Book Titles: Formatting Guide
| Last name, Initials. (Year of Publishing). Publisher. | Shakespeare, W. (1954). . The Folio Society. | |
| Last name, First Name. . Publisher. Year of publishing. | Shakespeare, William. . The Folio Society. 1954. | |
| Last name, First Name. . Publishing place: Publisher. Year of publishing. | Shakespeare, William. . London: The Folio Society. 1954. |
How to Write a Book Title in an Essay: APA
I’ll focus on the latest version, APA’s 7th edition [1]. For this format, you provide the author’s surname, initials, the publishing year (use round brackets), the italicized title, the publisher, and DOI if the manual has it.
If the book has several authors, place surnames in the alphabetic order, with “&” before the last surname. If it’s an edited work without an author, put the editor’s surname and initials at the beginning. In the case of several editors, add “Ed.” or “Eds.” after the initials.
If the copy has both an author and an editor, place the author’s surname initials in the beginning and add the editor’s surname and initials in brackets between the book title and the name of its publisher.
- Shakespeare, W. (1954). The tragedy of Hamlet, Prince of Denmark . The Folio Society.
- Black S. (1981). How to live this life . (White A. & Brown L., Eds.) Scale University Press.
How to Write a Book Title in an Essay: MLA
I provide the rules according to MLA’s 8th edition [2].
Provide the author’s full name, italicize the book title, the publishing place (for works older than 1900), the publisher, and the publication date.
If the copy has several authors , you write the first author’s surname and their name after a comma and then name other authors in the usual name-surname order. Add “and” before the last author in the list.
In the case of books with no authors, the MLA format doesn’t require you to note the editors. You can skip the part with authorship and start with the title in italics.
Examples to correctly cite books in MLA:
- Shakespeare, William. The tragedy of Hamlet, Prince of Denmark . The Folio Society. 1954.
- Encyclopedia of Montana . Summerset, 1993.
How to Write Book Titles in Essays: Chicago
The fine points of the Chicago style are the fine points for the footnotes and endnotes as in-text references:
In the footnote, you provide the author’s first and last name, italicize the book title, specify the place of publication, publisher, and year in round brackets, and write the page number.
The difference between the footnote and source in the bibliography is not so big. In the bibliography, you provide the author’s surname, not the name first. Also, don’t use round brackets and page numbers.
| William Shakespeare. . (London: The Folio Society. 1954), 6. | |
| Shakespeare, William. . London: The Folio Society. 1954. |
Writing Author Names in Paper Bodies
Authors’ surnames in the bodies of papers go in capital letters. Depending on the citation style, it might or might not require a personal name.
For example, APA 7 or MLA 8 doesn’t require the author’s name. In-text citations in APA will be “As Shakespeare (1954) writes, ” while Chicago, as you can see from above, requires both first and last names in footnotes.
In Lieu of a Conclusion: FAQs
- Are books italicized?
Yes, the titles of the books go in italics in the references.
- Do you underline book titles?
No, you don’t need to underline the book titles. The exception might be if you write an essay by hand. It’s hard to italicize titles when handwriting, so you underline the name to specify it.
- How do you write book titles with subtitles in an essay?
Italicize the title, start with a capital letter, and place the subtitle after the colon, also capitalized. For example : I am Malala: The story of the girl who stood up for education and was shot by the Taliban.
- How to write a short story title in an essay?
A story, poem, essay, or any other short genre represented as a book part must go in quotation marks. For example: “The Intruder.” It’s a short story in Andre Dubus’s collection, Dancing After Hours .
- Why properly cite books in essays?
The title of the books in the essay is critical for a reader to understand what kind of source this is.
References:
- https://owl.purdue.edu/owl/research_and_citation/index.html
2. https://libguides.southernct.edu/mla/core/title
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
How to Write A BOOK Title In An Essay

Writing a book title in an essay can be confusing. But it is necessary for the credibility and clarity of the write-up. Plus, each writing style has its own rules for formatting titles. Hence, doing such an activity could be a real pain for the students.
Don’t worry, as you are in the right place! Since this interesting article focuses on guiding you about how to write a book title in an essay accurately. So, read it thoroughly before you search for a professional paper writing services provider.
Table of Contents
Understanding Formatting Guidelines
The first step in learning how to write book name in essay is to learn the basics. It means you need to get comfortable with different formatting guidelines. Let’s begin with the style guides.
Different style guides
When writing essays for college , it’s important to know the rules for formatting book titles. The three most popular style guides are MLA, APA, and Chicago.
In MLA format , you should usually italicize book titles. You can also put them in quotation marks when a type of work demands.
For example, a book title like “To Kill a Mockingbird” would be italicized: To Kill a Mockingbird .
However, a chapter title within a book would be placed within quotation marks. For example, “The Ewell Family.”
In APA style , the first word of book titles is capital.
For example, a book title like “The Catcher in the Rye” would be written as The catcher in the rye
Chicago Style
Chicago style demands a book title to be in italics or quotation marks. It is very similar to the MLA style. But Chicago style gives you a bit more leeway to use italics or quotation marks. It’s best to stay consistent with what you pick throughout your essay when using the Chicago style.
Consistency within the Essay
You must be consistent when including the title of a book in an essay. Figure out what style guide you must follow and ensure you stick with it. That means all the book titles you mention should look the same.
For example, if you choose to italicize book titles according to MLA style. Ensure that all book titles in your essay are italicized consistently. Avoid mixing italicization with quotation marks or using different formatting styles within the same essay.
Inconsistency in formatting can confuse readers and undermine the professionalism of your work. Paying attention to detail and maintaining consistency will contribute to your essay’s overall clarity and readability.
Determine the Appropriate Style Guide to Follow
To determine the appropriate style guide to follow for formatting book titles in your essay, consider the following:
Assignment Requirements
See if your teacher or the instructions for the assignment mention a certain style to go by. Stick to that, if they do, to ensure everything is consistent, and you meet the expectations.
Academic Discipline
Your field of study can affect which style guide you should use. For example, humanities and literature students usually use MLA style, while social sciences usually use APA style. It’s important to know what’s typical in your discipline to choose the right guide.
Formatting Book Titles in MLA Style
Humanities and liberal arts disciplines use MLA writing rules. In MLA style, book titles are usually in italics like in APA style. But there can be variations in capitalization and punctuation. Let’s explore each aspect in detail with examples:
In MLA style, book titles are put in italics to make them stand out from the rest of the text.
Titles of shorter works, such as articles or chapters, are enclosed in quotation marks.
Example 1: Italicized Book Title
Fitzgerald, F. Scott. The Great Gatsby .
Example 2: Book Chapter (In Quotation Marks)
Smith, John. “The Art of Persuasion.” Essays on Rhetoric.
Capitalization
In MLA style, follows the title case. It means keep the first letter of each word capital. Capitalize articles, conjunctions, and prepositions only if they are the first or last words in title.
Example 3: Correct Capitalization
Lee, Harper. To Kill a Mockingbird.
Punctuation
In MLA style, there should be no special punctuation like colons or periods between the main title and any subtitles. However, if the book’s title includes a subtitle, a colon should separate it from the main title.
Example 4: Book Title with Subtitle
Gladwell, Malcolm. Outliers: The Story of Success.
Edition and Volume Numbers
To refer to a certain book edition, add the edition number after the book title. If the book is part of a multi-volume work, indicate the volume number after the title as well.
Example 5: Edition and Volume Numbers
Johnson, Mary. Chemistry in Focus. 2nd ed.
Smith, Adam. The Wealth of Nations. Vol. 1.
Translated Titles
If the book you are citing is translated from another language, include the original title and the translator’s name in the citation.
Example 6: Translated Title
Kafka, Franz. The Metamorphosis. Translated by David Wyllie.
It’s important to remember that MLA style is always changing and being updated. So always refer to the latest edition of the MLA Handbook or your institution’s writing guidelines.
Formatting Book Titles in APA Style
Usually the social sciences disciplines use APA (American Psychological Association) style. Let’s look at how you must consider capitalization, punctuation and italics in this writing style.
Just capitalize the first word of any subtitles and proper nouns.
All other words, such as articles (a, an, the), conjunctions (and, but, or), and prepositions (in, on, at), are in lowercase.
Example 1:
“The Power of Habit: Why We Do What We Do in Life and Business”
In APA style, book titles are italicized to distinguish them from the rest of the text.
Do not italicize titles of shorter works, such as articles or chapters. Just enclose them in quotation marks.
Example 2: Italics
Here’s an example of an italicized book title:
The Catcher in the Rye
In APA style, there should be a colon (:) between the main title and any subtitle.
When citing a book title within the text of your paper, use title case and italicize it.
When including book titles in your reference list, use sentence case and italicize it.
Example 3: Punctuation
Here’s an example of proper punctuation and citation within the text and reference list:
In-text citation
According to Smith (2019), The Theory of Everything provides an in-depth analysis of astrophysics.
Reference list citation
Smith, J. (2019). the theory of everything . Publisher.
Include the edition number in parentheses right after the book title when a book has a specific edition.
If a book is part of a multi-volume work, you can also indicate the volume number after the title.
Example 4: Parenthesis
Here are examples of how to format book titles with edition and volume numbers:
Edition Number
Johnson, M. (2022). Chemistry in Focus (2nd ed.).
Volume Number
Smith, A. (2021). History of the United States (Vol. 3).
Include the translator’s name in square brackets if you cite a translated book.
Example 5: Translated Thesis
Here’s an example of how to format a translated book title:
Kundera, M. (1984). The Unbearable Lightness of Being [Original title: Nesnesitelná lehkost bytí].
Translated by M. Henry.
Formatting Book Titles in Chicago Style
The Chicago Manual of Style is mostly used in the humanities and social sciences disciplines. Chicago style follows two systems, namely Author-Date System and the notes and bibliography system. Let’s explore both of them.
Author-Date System
In the author-date system, you include:
- In-text citations with the author’s last name
- The publication year
- A corresponding entry in the reference list
Italicization
In the author-date system, book titles are italicized. It makes them Distinguish from other elements in the citation.
Chicago style uses a title case for book titles in the author-date system. It means the first letter of the title, subtitles, and any major words are capitalized.
There should be a period at the end of the full book citation in the reference list.
Example 1: In-Text Citation
Example 2: Reference List Citation
Smith, John. 2019. The Theory of Everything . Publisher.
Notes and Bibliography System
You use footnotes or endnotes in the notes and bibliography system for in-text citations and a bibliography for the full list of references.
Similar to the author-date system, book titles are italicized in the notes and bibliography system.
In the notes and bibliography system, the Chicago style uses headline-style capitalization for book titles. It means that the first letter of the first and last words of the title are capitalized.
Put a period at the end of each full bibliographic entry in the notes and bibliography system.
Example 3: Footnote/Endnote Citation
John Smith, The Theory of Everything (Publisher, 2019), 25.
Example 4: Bibliography Citation
Smith, John. The Theory of Everything . Publisher, 2019.
You may include the edition number after the title, and for multi-volume works, the volume number after the title.
Example 5: Edition Number
Johnson, Mary. Chemistry in Focus . 2nd ed.
Example 6: Volume Number
Smith, Adam. The Wealth of Nations . Vol. 1.
For translated works, include the original title and the translator’s name in the citation.
Example 7: Translated Title
Kafka, Franz. The Metamorphosis . Translated by David Wyllie.
Citation of Book Titles in Other Situations
Let’s highlight some unusual circumstances of including a title of book in essay. Starting with:
Book titles within quotations
If you’re citing a direct quote from a book in your essay, you may need to put the book title in quotes. Generally, you should use double quotation marks for this.
For example:
According to Mark Twain, “The secret of getting ahead is getting started.”
In the novel 1984, George Orwell explores the theme of government surveillance through the famous line, “Big Brother is watching you.”
By using double quotation marks, you indicate that the words within the quotation marks are taken directly from the book.
Book Titles in Footnotes or Endnotes
In academic writing, footnotes or endnotes can be added to give extra info or credits. When including book titles, how you format them depends on the citation style you’re using.
In Chicago Style, book titles in footnotes or endnotes should usually be italicized or in quotation marks.
For Example:
Jane Austen, Pride and Prejudice (New York: Penguin Classics, 2002), 45.
Harper Lee, To Kill a Mockingbird , (New York: Harper Perennial, 2006), 77.
Handling Foreign language book titles
Follow these rules for citing a book in a foreign language. You should keep the original language title, especially if it’s a popular work.
Italicize the foreign language book title following the same guidelines as you would for an English book title. Include a translation in parentheses if necessary.
Use the original foreign language title in sentence case without italics or quotation marks. Include a translation in brackets if needed.
Italicize or use quotation marks for foreign language book titles, following the same guidelines as you would for an English book title. Include a translation if required.
Special Cases
In certain situations, you might need to format book titles differently. Like if you’re talking about a poem or play. These types of works have their own rules for formatting titles. Let’s get to know them briefly.
Typically, you’d put poem titles in quotation marks and longer pieces of poetry, like epics, in italics. It’s worth checking the style guide you’re using, though, since the rules can vary.
You’ll usually see the title written in italics when it comes to plays. The names of characters or speakers within the play are usually written with a mix of upper- and lowercase letters, without quotation marks.
Best Practices for Including Book Titles in Essays
Double-check formatting guidelines.
It’s super important to double-check the formatting rules for book titles when writing an essay since each style guide has its own rules. You need to make sure you’re following them properly.
Proofreading for Accuracy and Consistency
Look out for mistakes in how you’ve done the capitals, italics, and quotes. Double-check any extra rules that might apply to foreign language books, poems, plays, and other special cases.
Seek Assistance from Style Guides or Writing Resources
It’s a good idea to get help from style guides or writing tools when you are stuck with citations. You can also buy cheap essay from a well-reputed writing services provider.
It’s super important to get book titles in essays right. Not just for clarity but also to show you’re a pro. Ensure that you stick to the accurate style guide. It could be MLA, APA, or Chicago. Plus, there are special rules for poems and more.
Furthermore, if you need a professional to help you out with citations, do count on the expertise of our writers . They are always available to get you out of your troubles of how to write book titles in essays.
Do I need to include book titles in my essay?
How do i format book titles in mla style, should i italicize or use quotation marks for book titles in apa style.
Order Original Papers & Essays
Your First Custom Paper Sample is on Us!
Timely Deliveries
No Plagiarism & AI
100% Refund
Try Our Free Paper Writing Service
Related blogs.

Connections with Writers and support
Privacy and Confidentiality Guarantee
Average Quality Score
Frequently asked questions
How do you write a book title in mla.
In MLA style , book titles appear in italics, with all major words capitalized. If there is a subtitle, separate it from the main title with a colon and a space (even if no colon appears in the source). For example:
The format is the same in the Works Cited list and in the text itself. However, when you mention the book title in the text, you don’t have to include the subtitle.
The title of a part of a book—such as a chapter, or a short story or poem in a collection—is not italicized, but instead placed in quotation marks.
Frequently asked questions: MLA Style
In MLA style , footnotes or endnotes can be used to provide additional information that would interrupt the flow of your text.
This can be further examples or developments of ideas you only briefly discuss in the text. You can also use notes to provide additional sources or explain your citation practice.
You don’t have to use any notes at all; only use them to provide relevant information that complements your arguments or helps the reader to understand them.
No, you should use parenthetical MLA in-text citations to cite sources. Footnotes or endnotes can be used to add extra information that doesn’t fit into your main text, but they’re not needed for citations.
If you need to cite a lot of sources at the same point in the text, though, placing these citations in a note can be a good way to avoid cluttering your text.
According to MLA format guidelines, the Works Cited page(s) should look like this:
- Running head containing your surname and the page number.
- The title, Works Cited, centered and in plain text.
- List of sources alphabetized by the author’s surname.
- Left-aligned.
- Double-spaced.
- 1-inch margins.
- Hanging indent applied to all entries.
The MLA Works Cited lists every source that you cited in your paper. Each entry contains the author , title , and publication details of the source.
No, in an MLA annotated bibliography , you can write short phrases instead of full sentences to keep your annotations concise. You can still choose to use full sentences instead, though.
Use full sentences in your annotations if your instructor requires you to, and always use full sentences in the main text of your paper .
If you’re working on a group project and therefore need to list multiple authors for your paper , MLA recommends against including a normal header . Instead, create a separate title page .
On the title page, list each author on a separate line, followed by the other usual information from the header: Instructor, course name and number, and submission date. Then write the title halfway down the page, centered, and start the text of the paper itself on the next page.
Usually, no title page is needed in an MLA paper . A header is generally included at the top of the first page instead. The exceptions are when:
- Your instructor requires one, or
- Your paper is a group project
In those cases, you should use a title page instead of a header, listing the same information but on a separate page.
When an online source (e.g. web page , blog post) doesn’t list a publication date , you should instead list an access date .
Unlike a publication date, this appears at the end of your MLA Works Cited entry, after the URL, e.g. “A Complete Guide to MLA Style.” Scribbr , www.scribbr.com/category/mla/. Accessed 28 Mar. 2021 .
For offline sources with no publication date shown, don’t use an access date—just leave out the date.
The level of detail you provide in a publication date in your Works Cited list depends on the type of source and the information available. Generally, follow the lead of the source—if it gives the full date, give the full date; if it gives just the year, so should you.
Books usually list the year, whereas web pages tend to give a full date. For journal articles , give the year, month and year, or season and year, depending on what information is available. Check our citation examples if you’re unsure about a particular source type.
In an MLA Works Cited list , the names of months with five or more letters are abbreviated to the first three letters, followed by a period. For example, abbreviate Feb., Mar., Apr., but not June, July.
In the main text, month names should never be abbreviated.
In your MLA Works Cited list , dates are always written in day-month-year order, with the month abbreviated if it’s five or more letters long, e.g. 5 Mar. 2018.
In the main text, you’re free to use either day-month-year or month-day-year order, as long as you use one or the other consistently. Don’t abbreviate months in the main text, and use numerals for dates, e.g. 5 March 2018 or March 5, 2018.
In most standard dictionaries , no author is given for either the overall dictionary or the individual entries, so no author should be listed in your MLA citations.
Instead, start your Works Cited entry and your MLA in-text citation with the title of the entry you’re citing (i.e. the word that’s being defined), in quotation marks.
If you cite a specialist dictionary that does list an author and/or overall editor, these should be listed in the same way as they would for other citations of books or book chapters .
Some source types, such as books and journal articles , may contain footnotes (or endnotes) with additional information. The following rules apply when citing information from a note in an MLA in-text citation :
- To cite information from a single numbered note, write “n” after the page number, and then write the note number, e.g. (Smith 105n2)
- To cite information from multiple numbered notes, write “nn” and include a range, e.g. (Smith 77nn1–2)
- To cite information from an unnumbered note, write “un” after the page number, with a space in between, e.g. (Jones 250 un)
If you cite multiple Shakespeare plays throughout your paper, the MLA in-text citation begins with an abbreviated version of the title (as shown here ), e.g. ( Oth. 1.2.4). Each play should have its own Works Cited entry (even if they all come from the same collection).
If you cite only one Shakespeare play in your paper, you should include a Works Cited entry for that play, and your in-text citations should start with the author’s name , e.g. (Shakespeare 1.1.4).
No, do not use page numbers in your MLA in-text citations of Shakespeare plays . Instead, specify the act, scene, and line numbers of the quoted material, separated by periods, e.g. (Shakespeare 3.2.20–25).
This makes it easier for the reader to find the relevant passage in any edition of the text.
When an article (e.g. in a newspaper ) appears on non-consecutive pages (e.g. starting on page 1 and continuing on page 6), you should use “pp.” in your Works Cited entry, since it’s on multiple pages, but MLA recommends just listing the first page followed by a plus sign, e.g. pp. 1+.
In an MLA style Works Cited entry for a newspaper , you can cite a local newspaper in the same way as you would a national one, except that you may have to add the name of the city in square brackets to clarify what newspaper you mean, e.g. The Gazette [Montreal].
Do not add the city name in brackets if it’s already part of the newspaper’s name, e.g. Dallas Observer .
MLA doesn’t require you to list an author for a TV show . If your citation doesn’t focus on a particular contributor, just start your Works Cited entry with the title of the episode or series, and use this (shortened if necessary) in your MLA in-text citation .
If you focus on a particular contributor (e.g. the writer or director, a particular actor), you can list them in the author position , along with a label identifying their role.
It’s standard to list the podcast’s host in the author position , accompanied by the label “host,” in an MLA Works Cited entry. It’s sometimes more appropriate to use the label “narrator,” when the podcast just tells a story without any guests.
If your citation of the podcast focuses more on the contribution of someone else (e.g. a guest, the producer), they can be listed in the author position instead, with an appropriate label.
MLA recommends citing the original source wherever possible, rather than the source in which it is quoted or reproduced.
If this isn’t possible, cite the secondary source and use “qtd. in” (quoted in) in your MLA in-text citation . For example: (qtd. in Smith 233)
If a source is reproduced in full within another source (e.g. an image within a PowerPoint or a poem in an article ), give details of the original source first, then include details of the secondary source as a container. For example:
When you want to cite a PowerPoint or lecture notes from a lecture you viewed in person in MLA , check whether they can also be accessed online ; if so, this is the best version to cite, as it allows the reader to access the source.
If the material is not available online, use the details of where and when the presentation took place.
In an MLA song citation , you need to give some sort of container to indicate how you accessed the song. If this is a physical or downloaded album, the Works Cited entry should list the album name, distributor, year, and format.
However, if you listened to the song on a streaming service, you can just list the site as a container, including a URL. In this case, including the album details is optional; you may add this information if it is relevant to your discussion or if it will help the reader access the song.
When citing a song in MLA style , the author is usually the main artist or group that released the song.
However, if your discussion focuses on the contributions of a specific performer, e.g. a guitarist or singer, you may list them as author, even if they are not the main artist. If you’re discussing the lyrics or composition, you may cite the songwriter or composer rather than a performer.
When a source has no title , this part of your MLA reference is replaced with a description of the source, in plain text (no italics or quotation marks, sentence-case capitalization).
Whenever you refer to an image created by someone else in your text, you should include a citation leading the reader to the image you’re discussing.
If you include the image directly in your text as a figure , the details of the source appear in the figure’s caption. If you don’t, just include an MLA in-text citation wherever you mention the image, and an entry in the Works Cited list giving full details.
In MLA Style , you should cite a specific chapter or work within a book in two situations:
- When each of the book’s chapters is written by a different author.
- When the book is a collection of self-contained works (such as poems , plays , or short stories ), even if they are all written by the same author.
If you cite multiple chapters or works from the same book, include a separate Works Cited entry for each chapter.
If a source has no author, start the MLA Works Cited entry with the source title . Use a shortened version of the title in your MLA in-text citation .
If a source has no page numbers, you can use an alternative locator (e.g. a chapter number, or a timestamp for a video or audio source) to identify the relevant passage in your in-text citation. If the source has no numbered divisions, cite only the author’s name (or the title).
If you already named the author or title in your sentence, and there is no locator available, you don’t need a parenthetical citation:
- Rajaram argues that representations of migration are shaped by “cultural, political, and ideological interests.”
- The homepage of The Correspondent describes it as “a movement for radically different news.”
If a source has two authors, name both authors in your MLA in-text citation and Works Cited entry. If there are three or more authors, name only the first author, followed by et al.
| Number of authors | In-text citation | Works Cited entry |
|---|---|---|
| 1 author | (Moore 37) | Moore, Jason W. |
| 2 authors | (Moore and Patel 37) | Moore, Jason W., and Raj Patel. |
| 3+ authors | (Moore et al. 37) | Moore, Jason W., et al. |
You must include an MLA in-text citation every time you quote or paraphrase from a source (e.g. a book , movie , website , or article ).
MLA Style is the second most used citation style (after APA ). It is mainly used by students and researchers in humanities fields such as literature, languages, and philosophy.
If information about your source is not available, you can either leave it out of the MLA citation or replace it with something else, depending on the type of information.
- No author : Start with the source title.
- No title : Provide a description of the source.
- No date : Provide an access date for online sources; omit for other sources.
A standard MLA Works Cited entry is structured as follows:
Only include information that is available for and relevant to your source.
Yes. MLA style uses title case, which means that all principal words (nouns, pronouns , verbs, adjectives , adverbs , and some conjunctions ) are capitalized.
This applies to titles of sources as well as the title of, and subheadings in, your paper. Use MLA capitalization style even when the original source title uses different capitalization .
The title of an article is not italicized in MLA style , but placed in quotation marks. This applies to articles from journals , newspapers , websites , or any other publication. Use italics for the title of the source where the article was published. For example:
Use the same formatting in the Works Cited entry and when referring to the article in the text itself.
In MLA style citations , format a DOI as a link, including “https://doi.org/” at the start and then the unique numerical code of the article.
DOIs are used mainly when citing journal articles in MLA .
The MLA Handbook is currently in its 9th edition , published in 2021.
This quick guide to MLA style explains the latest guidelines for citing sources and formatting papers according to MLA.
The fastest and most accurate way to create MLA citations is by using Scribbr’s MLA Citation Generator .
Search by book title, page URL, or journal DOI to automatically generate flawless citations, or cite manually using the simple citation forms.
MLA recommends using 12-point Times New Roman , since it’s easy to read and installed on every computer. Other standard fonts such as Arial or Georgia are also acceptable. If in doubt, check with your supervisor which font you should be using.
To create a correctly formatted block quote in Microsoft Word, follow these steps:
- Hit Enter at the beginning and end of the quote.
- Highlight the quote and select the Layout menu.
- On the Indent tab, change the left indent to 0.5″.
Do not put quotation marks around the quote, and make sure to include an MLA in-text citation after the period at the end.
To format a block quote in MLA:
- Introduce the quote with a colon and set it on a new line.
- Indent the whole quote 0.5 inches from the left margin.
- Place the MLA in-text citation after the period at the end of the block quote.
Then continue your text on a new line (not indented).
In MLA style , if you quote more than four lines from a source, use MLA block quote formatting .
If you are quoting poetry , use block quote formatting for any quote longer than three lines.
An MLA in-text citation should always include the author’s last name, either in the introductory text or in parentheses after a quote .
If line numbers or page numbers are included in the original source, add these to the citation.
If you are discussing multiple poems by the same author, make sure to also mention the title of the poem (shortened if necessary). The title goes in quotation marks .
In the list of Works Cited , start with the poet’s name and the poem’s title in quotation marks. The rest of the citation depends on where the poem was published.
If you read the poem in a book or anthology, follow the format of an MLA book chapter citation . If you accessed the poem online, follow the format of an MLA website citation .
Only use line numbers in an MLA in-text citation if the lines are numbered in the original source. If so, write “lines” in the first citation of the poem , and only the numbers in subsequent citations.
If there are no line numbers in the source, you can use page numbers instead. If the poem appears on only one page of a book (or on a website ), don’t include a number in the citation.
To quote poetry in MLA style , introduce the quote and use quotation marks as you would for any other source quotation .
If the quote includes line breaks, mark these using a forward slash with a space on either side. Use two slashes to indicate a stanza break.
If the quote is longer than three lines, set them off from the main text as an MLA block quote . Reproduce the line breaks, punctuation, and formatting of the original.
Ask our team
Want to contact us directly? No problem. We are always here for you.
- Email [email protected]
- Start live chat
- Call +1 (510) 822-8066
- WhatsApp +31 20 261 6040

Our team helps students graduate by offering:
- A world-class citation generator
- Plagiarism Checker software powered by Turnitin
- Innovative Citation Checker software
- Professional proofreading services
- Over 300 helpful articles about academic writing, citing sources, plagiarism, and more
Scribbr specializes in editing study-related documents . We proofread:
- PhD dissertations
- Research proposals
- Personal statements
- Admission essays
- Motivation letters
- Reflection papers
- Journal articles
- Capstone projects
Scribbr’s Plagiarism Checker is powered by elements of Turnitin’s Similarity Checker , namely the plagiarism detection software and the Internet Archive and Premium Scholarly Publications content databases .
The add-on AI detector is powered by Scribbr’s proprietary software.
The Scribbr Citation Generator is developed using the open-source Citation Style Language (CSL) project and Frank Bennett’s citeproc-js . It’s the same technology used by dozens of other popular citation tools, including Mendeley and Zotero.
You can find all the citation styles and locales used in the Scribbr Citation Generator in our publicly accessible repository on Github .
- Flashes Safe Seven
- FlashLine Login
- Faculty & Staff Phone Directory
- Emeriti or Retiree
- All Departments
- Maps & Directions

- Building Guide
- Departments
- Directions & Parking
- Faculty & Staff
- Give to University Libraries
- Library Instructional Spaces
- Mission & Vision
- Newsletters
- Circulation
- Course Reserves / Core Textbooks
- Equipment for Checkout
- Interlibrary Loan
- Library Instruction
- Library Tutorials
- My Library Account
- Open Access Kent State
- Research Support Services
- Statistical Consulting
- Student Multimedia Studio
- Citation Tools
- Databases A-to-Z
- Databases By Subject
- Digital Collections
- Discovery@Kent State
- Government Information
- Journal Finder
- Library Guides
- Connect from Off-Campus
- Library Workshops
- Subject Librarians Directory
- Suggestions/Feedback
- Writing Commons
- Academic Integrity
- Jobs for Students
- International Students
- Meet with a Librarian
- Study Spaces
- University Libraries Student Scholarship
- Affordable Course Materials
- Copyright Services
- Selection Manager
- Suggest a Purchase
Library Locations at the Kent Campus
- Architecture Library
- Fashion Library
- Map Library
- Performing Arts Library
- Special Collections and Archives
Regional Campus Libraries
- East Liverpool
- College of Podiatric Medicine
- Kent State University
- APA Style - 7th edition
- Specific Rules for Authors & Titles
APA Style - 7th edition: Specific Rules for Authors & Titles
- Basic Information
Rules for Writing Author and Editor Information
Rules for writing titles.
- Media Sources
- Internet Sources
- In-text Citations
- Reference Lists
There are certain things to keep in mind when writing the author's name according to APA style. Authors may be individual people, multiple people, groups (institutions or organizations), or a combination of people and groups.
- You must include all the authors up to 20 for individual items. For example, if you are using an article that has 19 authors you must list them all out on your reference page.
- Use initials for the first and middle names of authors. Use one space between initials.
- All names are inverted (last name, first initial).
- Do not hyphenate a name unless it is hyphenated on the item.
- Separate the author's names with a comma and use the ampersand symbol "&" before the last author listed.
- Spell out the name of any organization that is listed as an author.
- If there is no author listed, the item title moves in front of the publication date and is used.
An item that you use may have an editor instead of an author or in the case of audiovisual materials a writer or director.
- For editors follow the same rules above and put the abbreviation (Ed.) or (Eds.) behind the name(s).
- For audiovisual materials follow the same rules as above and put the specialized role (Writer) (Director) behind the name.
Zhang, Y. H. (one author)
Arnec, A., & Lavbic, D. (two authors)
Kent State University (organization as author)
Barr, M. J. (Ed.). (1 editor)
Powell, R. R., & Westbrook, L. (Eds.). (2 editors)
here are certain things to keep in mind when writing a title according to APA style.
- Book titles are italicized and written using sentence case (only the first word of a title, subtitle, or proper noun are capitalized).
- Book chapter titles are written using sentence case and are not italicized.
- Journal titles are italicized and written using title case (all the important words are capitalized).
- Article titles are written using sentence case and are not italicized.
- Webpages and websites are italicized and written using sentence case.
Publication manual of the American Psychological Association (book title, American Psychological Association is a proper noun so it is capitalized)
Student perspective of plagiarism (book chapter title)
Internet plagiarism in higher education: Tendencies, trigging factors and reasons among teacher candidates (article title, Tendencies is the first word of a sub-title so it is capitalized)
Assessment & Evaluation in Higher Education (journal title)
- << Previous: Basic Information
- Next: Books >>
- Last Updated: Jul 14, 2023 4:23 PM
- URL: https://libguides.library.kent.edu/apa7
Street Address
Mailing address, quick links.
- How Are We Doing?
- Student Jobs
Information
- Accessibility
- Emergency Information
- For Our Alumni
- For the Media
- Jobs & Employment
- Life at KSU
- Privacy Statement
- Technology Support
- Website Feedback
- PRO Courses Guides New Tech Help Pro Expert Videos About wikiHow Pro Upgrade Sign In
- EDIT Edit this Article
- EXPLORE Tech Help Pro About Us Random Article Quizzes Request a New Article Community Dashboard This Or That Game Popular Categories Arts and Entertainment Artwork Books Movies Computers and Electronics Computers Phone Skills Technology Hacks Health Men's Health Mental Health Women's Health Relationships Dating Love Relationship Issues Hobbies and Crafts Crafts Drawing Games Education & Communication Communication Skills Personal Development Studying Personal Care and Style Fashion Hair Care Personal Hygiene Youth Personal Care School Stuff Dating All Categories Arts and Entertainment Finance and Business Home and Garden Relationship Quizzes Cars & Other Vehicles Food and Entertaining Personal Care and Style Sports and Fitness Computers and Electronics Health Pets and Animals Travel Education & Communication Hobbies and Crafts Philosophy and Religion Work World Family Life Holidays and Traditions Relationships Youth
- Browse Articles
- Learn Something New
- Quizzes Hot
- This Or That Game
- Train Your Brain
- Explore More
- Support wikiHow
- About wikiHow
- Log in / Sign up
- Education and Communications
- College University and Postgraduate
- Academic Writing
- MLA Style Manual
How to Write Book Titles in MLA
Last Updated: April 1, 2024 Fact Checked
This article was co-authored by Annaliese Dunne and by wikiHow staff writer, Jennifer Mueller, JD . Annaliese Dunne is a Middle School English Teacher. With over 10 years of teaching experience, her areas of expertise include writing and grammar instruction, as well as teaching reading comprehension. She is also an experienced freelance writer. She received her Bachelor's degree in English. There are 8 references cited in this article, which can be found at the bottom of the page. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed 49,062 times.
If you're writing a research paper, you may want to include the title of a book in the text of your paper. The handbook for the Modern Language Association (MLA) provides specific rules for how to set off the title of a book from the rest of your text. Generally, titles of books are set apart from the rest of the text following particular formatting, capitalization, and punctuation rules.
Formatting Titles

- Music album titles and film titles are also italicized.
- In earlier editions, the MLA Handbook also permitted titles to be underlined. The 8th edition confirms that underlining is no longer appropriate.
Exception : Religious texts, such as the Bible or the Quran, are not italicized.

- For example, you might write: "In The Great Gatsby in the Classroom: Searching for the American Dream , David Dowling promotes the use of art and film to improve literacy."

- For example, if you were talking about the Nancy Drew series, you would not italicize "Nancy Drew" because the name does not appear in the titles of any of the individual books. In contrast, Harry Potter would be italicized, because the name appears at the beginning of the title of every book in the series.

- For example, if you were writing a paper on The Great Gatsby , you would need to include the full title in your text at least once. If you mentioned the novel more than once, you could use Gatsby as a shortened title.
- How you shorten the title generally depends on your own judgment. Use a word or words that evoke the title easily in the mind of the reader, without any ambiguity.
Capitalizing Titles

- For example, you might write: "Comedian Steve Martin takes on the art world in his novel An Object of Beauty . Note that the word "an" is capitalized because it is the first word. Otherwise, it would be in lowercase.
- If the book also has a subtitle, capitalize the first word of the subtitle just as you did the first word of the title.

- Articles include words such as "a," "an," and "the." Words such as "against," "in," and "to." Note that these words remain in lowercase regardless of their length.

- Coordinating conjunctions include words such as "and," "but," "for," and "nor."
- Subordinating conjunctions include words such as "after," "although," because," "unless," and "until."

- Example: Storytelling and Mythmaking: Images from Film and Literature
Tip: If you have doubts about the parts of speech of the words in a title, you can check your capitalization using free capitalization checkers available online.

- Capitalize all words that would be capitalized in the language the title is written in. For example, since all nouns are capitalized in German, you would capitalize all nouns in a German book title.
Punctuating Titles

- For example, if a title ends in an exclamation point or a question mark, you would include that punctuation mark at the end of the title. The punctuation mark should be italicized so that your readers understand that it's part of the title, not part of your punctuation.
- If the book title ends in a question mark or an exclamation point, it's typically not a good idea to use that title at the end of a sentence, because then the punctuation mark at the end of the book title effectively becomes the punctuation mark for the end of your sentence. Recast your sentence so that the book title doesn't fall at the end of the sentence.

- There is an exception if the book title includes another book title, and the included book title ends in a question mark or exclamation point. Since that punctuation mark belongs to the included book title, you would follow that punctuation mark with a colon. For example: Moby Dick and Absalom, Absalom! : Two American Masterpieces Note that the colon is italicized.

- For example, you might write: "In the 1980s sitcom When Harry Met Sally. . . , the characters examined whether heterosexual cisgender men and women could be friends without ever becoming romantically or sexually involved."
- Note that you'll have to form your sentence so that either a comma or a period is appropriate following the ellipsis or a dash.
Tip: Although it might be difficult for the average person to tell whether a punctuation mark such as a comma or period is italicized, technically any punctuation added to the end of the title should not be italicized.

- For example: England's Monitor; or, The History of Separation . Note that the "or" and all punctuation are also italicized.
- Generally, list the original or oldest title first, followed by any later title.

- For example, since the book Everything Is Nothing: The Poetry of the Great War, Revolution and the Transformation of Europe was published in London, the serial comma would not be added. However, if the book were published in the US, you would add a comma after the word "Revolution."
Expert Q&A
You might also like.

Expert Interview

Thanks for reading our article! If you’d like to learn more about writing, check out our in-depth interview with Annaliese Dunne .
- ↑ https://owl.purdue.edu/owl/subject_specific_writing/writing_in_literature/writing_about_literature/formatting.html
- ↑ https://style.mla.org/punctuation-with-titles/
- ↑ https://style.mla.org/trilogy-titles/
- ↑ https://style.mla.org/full-names-titles-in-each-chapter/
- ↑ https://secondary.oslis.org/cite-sources/mla/how-to-capitalize-and-punctuate-titles
- ↑ https://style.mla.org/capitalization-of-titles/
- ↑ https://irsc.libguides.com/c.php?g=483085&p=3303403
- ↑ https://morningside.libguides.com/MLA8/title
About This Article

- Send fan mail to authors
Did this article help you?

Featured Articles

Trending Articles

Watch Articles

- Terms of Use
- Privacy Policy
- Do Not Sell or Share My Info
- Not Selling Info
Get all the best how-tos!
Sign up for wikiHow's weekly email newsletter
- Western Libraries
- Ask Us! Answer Service
Q. How do I refer to a book by title in-text in APA format?
- Research & Writing Studio
- 21 Accounts
- 14 Acquisitions
- 4 Anthropology
- 71 APA citations and formatting
- 35 Archives
- 31 Archives & Special Collections
- 36 Articles
- 14 Business resources
- 11 Center for Pacific Northwest Studies
- 3 Chemistry
- 8 Chicago citations and formatting
- 85 Circulation Services (check out/return/renew items)
- 42 Citations and style guides
- 44 Collections
- 50 Community services
- 1 Computer science
- 38 Computers
- 47 Copyright
- 79 Databases
- 22 Digital collections
- 87 Directions
- 7 Education (studies)
- 3 Engineering
- 2 English literature
- 7 Environmental studies/sciences
- 23 Equipment
- 42 Faculty services
- 3 Fairhaven
- 9 Fines and fees
- 12 Fun facts
- 21 Government information
- 5 Graduate students
- 2 Grant writing
- 1 Guest services
- 5 Human Services
- 50 Inter-library loan
- 17 Journals
- 29 Learning Commons
- 8 Library instruction
- 78 Library services
- 13 MLA citations and formatting
- 29 Multimedia
- 6 Newspapers
- 55 OneSearch
- 4 Online Learning
- 64 Outreach and Continuing Education
- 29 Policies
- 2 Political science
- 29 Primary sources
- 30 Printing related
- 3 Psychology
- 2 Rehabilitation Counseling
- 86 Research
- 17 Research & Writing Studio
- 37 Reserves
- 6 Scholarly communication
- 3 Sociology
- 10 Special Collections
- 1 Streaming video
- 44 Student services
- 28 Student Technology Center
- 1 Teaching and Learning Academy
- 16 Technology
- 3 Troubleshooting
- 5 Tutoring Center
- 5 Undergraduate Research Award
- 5 Undergraduate Students
- 18 Video tutorial
- 11 Western CEDAR
- 1 Women's Studies
- 37 Writing related
- 93 WWU general info
Answered By: Gabe Gossett Last Updated: Jun 22, 2023 Views: 645636
The basic format for an in-text citation is: Title of the Book (Author Last Name, year).
One author: Where the Wild Things Are (Sendak, 1963) is a depiction of a child coping with his anger towards his mom.
Two authors (cite both names every time): Brabant and Mooney (1986) have used the comic strip to examine evidence of sex role stereotyping. OR The comic strip has been used to examine evidence of sex role stereotyping (Brabant & Mooney, 1986).
Three or more authors (cite the first author plus et al.): Tales from the Shadowhunter Academy (Clare et al., 2016) depicts a young man's experience at the Shadowhunter Academy, a place where being a former vampire is looked down upon.OR Clare et al. (2016) have crafted a unique story about a young man's journey to find himself.
No author: Cite the first few words of the reference entry (usually the title) and the year. Use double quotation marks around the title of an article or chapter, and italicize the title of a periodical, book, brochure, or report. Examples: From the book Study Guide (2000) ... or ("Reading," 1999).
Note: Titles of periodicals, books, brochures, or reports should be in italics and use normal title capitalization rules.
If you are citing multiple sources by multiple authors in-text, you can list all of them by the author's last name and year of publication within the same set of parentheses, separated by semicolons.
Example: (Adams, 1999; Jones & James, 2000; Miller, 1999)
For more information on how to cite books in-text and as a reference entry, see the APA Publication Manual (7th edition) Section 10.2 on pages 321-325 .
Links & Files
- APA Workshop
- Citation Quick Guides and Style Manuals
- Share on Facebook
Was this helpful? Yes 111 No 86
Comments (13)
- This was very useful for me! I was having a really hard time finding information on how to mention an article title AND the author in text in APA so this was very helpful!!! by Ryan Waddell on Jun 27, 2019
- If I just mention that I used a book to teach a topic do I have to include it in the reference list? by Franw on Oct 17, 2019
- @Franw, if it is a source that informs your paper in any way, or if your reader would have reason to look it up, then you should include a full reference list entry for the book. by Gabe [Research & Writing Studio] on Oct 18, 2019
- Maybe I'm misunderstanding the question, but I think the OP is asking how to refer to a book title, not how to cite one. I believe APA uses quotation marks around book titles and MLA uses italics. by AB on Dec 12, 2019
- @AB: The first sentence has been tweaked to clarify title of book usage, reflecting the examples given. For APA style you should use italics for book titles. It would be quotation marks. by Gabe [Research & Writing Studio] on Dec 12, 2019
- Hi, can any one help me with in-text-citation of this, how can i cite it in the text Panel, I. L. (2002). Digital transformation: A framework for ICT literacy. Educational Testing Service, 1-53. by Milad on Aug 20, 2021
- @Milad: In that case it would be (Panel, 2002). If you are quoting, or otherwise choosing to include page numbers, put a comma after the year, then p. and the page number(s). by Gabe Gossett on Aug 20, 2021
- Hey, I'm a little bit curious, what if I'm mentioning a book and paraphrasing it but still want to give credit. Would I put the information into parenthesis instead? Like: Paraphrased info. ("Title in Italics" Author, year) by Kai on Sep 14, 2023
- @Kai: Apologies for not seeing your question sooner! (Our academic year has not started yet). If I am understanding your question correctly, what I suggest is referring to the book title in the narrative of your writing, rather than in the in-text citation. I do not see an examples of using a book title in an in-text citation except for rare circumstances including citing a classic religious text or using the title when there is no author information because it is the start of your reference list entry. Basically, APA's in-text convention is supposed to make it easy for your reader to locate the source being cited in the reference list. So the first part of the in-text citation, usually authors, comes first to locate it alphabetically. Putting the book title first when you have an author name can throw that off. by Gabe Gossett on Sep 21, 2023
- Perhaps this is along the lines of the response to Kai - Can you reference a book title as a common point of social understanding to demonstrate a common concept? Is official citing required if you use widely known titles such as "Where's Waldo" and "Who Moved My Cheese?" to make a point of illustration? by Chez Renee on Sep 30, 2023
- @Chez: Aside from some classical religious texts, if it is a published book, I'd try to make sure that it is appropriately cited for APA style. That said, I think I understand where it gets tricky with things like Where's Waldo, since that is a series of books and stating "Where's Waldo" is a cultural reference many people would understand, though you can't reasonably cite the entire series. I don't believe that APA gives guidance for this particular issue. If it is being referred to in order to back up a claim, it would help to cite a particular book. If not, then it might work to use a statement such as, "Hanford's Where's Waldo series . . ." by Gabe Gossett on Oct 02, 2023
- How to cite a dissertation thesis in apa form? by Elizabeth on Feb 05, 2024
- @Elizabeth: For citing a dissertation or thesis you can check out our page answering that here https://askus.library.wwu.edu/faq/153308 by Gabe Gossett on Feb 05, 2024
- Find the librarian for your subject area
Related Topics
- APA citations and formatting
- +44 (0) 207 391 9032
Recent Posts
- How to Structure Your Dissertation in 2024
How to Write a Research Paper Like a Pro
- Academic Integrity vs. Academic Dishonesty: Understanding the Key Differences
- How to Use AI to Enhance Your Thesis
- Guide to Structuring Your Narrative Essay for Success
- How to Hook Your Readers with a Compelling Topic Sentence
- Is a Thesis Writing Format Easy? A Comprehensive Guide to Thesis Writing
- The Complete Guide to Copy Editing: Roles, Rates, Skills, and Process
- How to Write a Paragraph: Successful Essay Writing Strategies
- Everything You Should Know About Academic Writing: Types, Importance, and Structure
- Academic News
- Custom Essays
- Dissertation Writing
- Essay Marking
- Essay Writing
- Essay Writing Companies
- Model Essays
- Model Exam Answers
- Oxbridge Essays Updates
- PhD Writing
- Significant Academics
- Student News
- Study Skills
- University Applications
- University Essays
- University Life
- Writing Tips

How to Title an Essay: Tips and Examples
(Last updated: 5 April 2024)
Since 2006, Oxbridge Essays has been the UK’s leading paid essay-writing and dissertation service
We have helped 10,000s of undergraduate, Masters and PhD students to maximise their grades in essays, dissertations, model-exam answers, applications and other materials. If you would like a free chat about your project with one of our UK staff, then please just reach out on one of the methods below.
Crafting an essay title is like designing the cover of a book – it's the first thing your professors see, setting the stage for what's inside. As such, the title is crucial because it's the reader's first impression. In this guide, we'll explore why a good essay title matters, offer tips to help you learn how to title an essay and provide examples to illustrate which titles work and which don't.
The Importance of a Good Essay Title
The significance of a good essay title cannot be overstated. Beyond its role as a mere label, a compelling title serves as a powerful tool for drawing your professor’s attention. A good title not only encapsulates the essence of the essay but also reflects the author's voice and perspective. It sets the tone for the entire piece, guiding readers in their interpretation and understanding of the content. Moreover, a well-crafted title can enhance the credibility and authority of the essay, signalling that the author has invested thought and care into their work.
How to Write a Good Title for Your Essay
Crafting a good title requires a blend of creativity, precision, and strategic thinking. To create an effective title, consider the following tips:
- Brainstorm Ideas : Begin by brainstorming keywords, phrases, and concepts related to your essay topic. Explore different angles and perspectives that encapsulate the essence of your argument or analysis.
- Consider the Audience : Reflect on your target audience and their interests, preferences, and expectations. Tailor your title to resonate with your intended readership, striking a balance between familiarity and intrigue.
- Capture the Essence : Reports often incorporate tables, charts, graphs, and other visual aids to enhance the presentation of data and facilitate understanding.
- Evoke Emotion or Intrigue : Tap into the emotional or intellectual curiosity of your readers by crafting a title that evokes emotion, prompts reflection, or poses a compelling question. Consider using provocative language, vivid imagery, or rhetorical devices to capture readers' attention.
- Revise and Refine: : Once you've generated potential titles, take time to revise and refine them. Experiment with different word choices, phrasings, and structures until you find a title that resonates and feels cohesive with the content of your essay.
Characteristics of a Good Title
A good title shares several key characteristics:
Descriptive : Clearly communicates the topic or main idea of the essay. Engaging : Captures the reader's attention and sparks curiosity. Concise : Succinctly summarises the content without being overly long or verbose. Relevant : Directly relates to the content and theme of the essay. Original : Avoids clichés and generic phrases. Try to strive for originality and creativity.
Essay Title Examples (Good vs. Bad)
Let's examine additional examples of good and bad essay titles to illustrate these principles:
Good Title : "Exploring Identity: The Intersection of Culture and Self-Perception" Bad Title : "Essay on Identity"
The good title invites readers to explore complex questions surrounding identity and self-perception, fostering curiosity and engagement. In contrast, the bad title lacks specificity and fails to capture the richness and depth of the essay's subject matter.
Good Title : "Breaking Barriers: The Evolution of Women's Rights in the 21st Century" Bad Title : "Essay on Women's Rights"
The good title is engaging and suggests a narrative, while the bad title is a little bland and lacks imagination.
Writing a Catchy Title for Your Essay
To craft a catchy title, consider employing the following strategies:
Wordplay and Alliteration : Incorporate puns, alliteration, or clever wordplay to make your title memorable and attention-grabbing.
Question or Provocation : Pose a thought-provoking question or statement that challenges readers' assumptions or prompts them to reconsider their perspectives.
Use of Imagery and Metaphor : Invoke vivid imagery or metaphorical language that evokes emotion, stimulates the senses, or conveys abstract concepts in concrete terms.
Surprise Element : Introduce an unexpected twist or element of surprise that captures readers' attention and leaves them eager to learn more.
Elevate Your Essay with An Engaging Title
Writing a good title for your essay requires time, skill, and a keen understanding of your subject matter. Crafting an essay title is more than just a formality—it's an art that can set the stage for the narrative that follows. With practice and attention to detail, you'll master the art of essay titling and unleash the full potential of your written creations.

Essay exams: how to answer ‘To what extent…’

How to write a master’s essay

Writing Services
- Essay Plans
- Critical Reviews
- Literature Reviews
- Presentations
- Dissertation Title Creation
- Dissertation Proposals
- Dissertation Chapters
- PhD Proposals
- Journal Publication
- CV Writing Service
- Business Proofreading Services
Editing Services
- Proofreading Service
- Editing Service
- Academic Editing Service
Additional Services
- Marking Services
- Consultation Calls
- Personal Statements
- Tutoring Services
Our Company
- Frequently Asked Questions
- Become a Writer
Terms & Policies
- Fair Use Policy
- Policy for Students in England
- Privacy Policy
- Terms & Conditions
- [email protected]
- Contact Form
Payment Methods
Cryptocurrency payments.

IMAGES
VIDEO
COMMENTS
Use capital letters to write the title of the novel. For example, The Secret Garden by Frances Hodgson Burnett. Use italics and capital letters to write the name of the author and his/her other works mentioned in a book title—for example, Jane Austen's Pride and Prejudice (1813). You should use quotation marks when writing headings of short ...
Exceptions to the Rule. The rule for writing book titles in italics applies specifically to running text. If the book title is standing on its own, as in a heading, there's no need to italicize it. Additionally, if the book is part of a larger series and you're mentioning both the title of the series and that of the individual book, you can ...
Heart of Darkness ). Place the name of a single chapter in quote marks, instead ("The Great Towns" from Condition of the Working Class in England by Friedrich Engels). APA. Italicize the book title. Capitalize the first letter, the first letter of a subtitle, and proper nouns.
The answer is: in this case, yes. In other cases, sometimes. It's really not as confusing as it seems. When you are talking about a book series but don't want or need to include the complete series titles for the purposes of your work, you only have to put words in italics that also appear in the book titles. So, because Harry Potter is ...
Book titles: Author names: Write the title in italicsDo not use quotation marks (unless you're speaking about the book's chapter, not the entire piece)Capitalize the first and last words, proper names, all significant words, and subordinating conjunctions (although, because, if, unless, etc.)Do not capitalize articles (a, the), prepositions (unless they come first or last), or coordinating ...
For example, you would write the name of William Faulkner's novel Absalom, Absalom! with both the comma and the exclamation point in italics. 4. Highlight the book name. Hover your cursor at the beginning of the book name and left click your mouse. Hold the key down and drag your cursor over the title of the book.
4. In-text Citation: Whenever you reference a book title within your essay, it's essential to provide an in-text citation to acknowledge the source. This citation typically includes the author's last name and the publication year in parentheses. For example, you might write, "In the novel '1984' (Orwell, 1949)…". 5.
How to Write a Book Title in an Essay: APA. Formatting template: Examples: Independent and self-contained books: ... Do you italicize book titles? Yes, you put book titles in italics. Please italicize long and stand-alone works: books, movies, webpages, reports, or music albums. Shorter works' titles (articles, essays, poems, songs, or book ...
Learn how to properly write a book title in an essay with this comprehensive step-by-step guide. Master the art of formatting and make your essays stand out. Login to comment; Logged in as . Contact. ... How to Gracefully Incorporate Book Titles into Your Essays: A Stylistic Approach ...
In fact, most style guides, including MLA and Chicago style, require book titles to be italicized, not underlined. If the book title has a subtitle, the subtitle should be italicized as well and separated by a colon to be formatted correctly for MLA style, as in: Natural History of the Intellect: the last lectures of Ralph Waldo Emerson.
Use quotation marks around the title if it is part of a larger work (e.g. a chapter of a book, an article in a journal, or a page on a website). All major words in a title are capitalized. The same format is used in the Works Cited list and in the text itself. When you use the Scribbr MLA Citation Generator, the correct formatting and ...
Capitalize the first word of titles of books in papers, the first word after a colon, and all major words. Avoid capitalizing minor words (e.g., articles, prepositions, conjunctions) unless they are the first word of the name or longer than four letters. Always place the book title after the author's name.
When citing a non-fiction book, use the same format as you would for a fictional work. Italicize the book title in the text and the Works Cited entry. Include the author's name, book title (in italics), publisher, year of publication, and medium of publication. For instance:
Provide the author's full name, italicize the book title, the publishing place (for works older than 1900), the publisher, and the publication date. If the copy has several authors, you write the first author's surname and their name after a comma and then name other authors in the usual name-surname order.
In APA style, there should be a colon (:) between the main title and any subtitle. When citing a book title within the text of your paper, use title case and italicize it. When including book titles in your reference list, use sentence case and italicize it. Example 3: Punctuation.
11. Write a one-word title—the most obvious one possible. 12. Write a less obvious one-word title. 13. Write a two-word title. 14. Write a three-word title. 15. Write a four-word title. 16. Write a five-word title. 17. Think of a familiar saying, or the title of a book, song, or movie, that might fit your essay. 18. Take the title you just ...
If a source has no author, start the MLA Works Cited entry with the source title.Use a shortened version of the title in your MLA in-text citation.. If a source has no page numbers, you can use an alternative locator (e.g. a chapter number, or a timestamp for a video or audio source) to identify the relevant passage in your in-text citation. If the source has no numbered divisions, cite only ...
Use initials for the first and middle names of authors. Use one space between initials. All names are inverted (last name, first initial). Do not hyphenate a name unless it is hyphenated on the item. Separate the author's names with a comma and use the ampersand symbol "&" before the last author listed. Spell out the name of any organization ...
Generally, list the original or oldest title first, followed by any later title. 5. Add the serial comma if necessary for books published in the US. Although the general rule is not to add any punctuation to a title that isn't included in the original work, the MLA Handbook makes an exception for the serial comma.
Jun 22, 2023 644613. The basic format for an in-text citation is: Title of the Book (Author Last Name, year). Examples. One author: Where the Wild Things Are (Sendak, 1963) is a depiction of a child coping with his anger towards his mom. Two authors (cite both names every time): Brabant and Mooney (1986) have used the comic strip to examine ...
Capitalize the first and final word of the title. Capitalize nouns, pronouns, verbs, helping verbs, adjectives and adverbs within the title. Capitalize the first word that follows a colon when using title case. Do not capitalize articles located between the first and final words, such as "the," "a" and "an."
Crafting a good title requires a blend of creativity, precision, and strategic thinking. To create an effective title, consider the following tips: Brainstorm Ideas: Begin by brainstorming keywords, phrases, and concepts related to your essay topic. Explore different angles and perspectives that encapsulate the essence of your argument or analysis.